Abstract
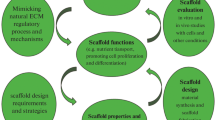
The need to shift from tissue replacement to tissue regeneration has led to the development of tissue engineering and in situ tissue regeneration. Both of these strategies often employ the use of scaffolds––templates that allow cells to attach and then guide the new tissue growth. There are many design criteria for an ideal scaffold. These criteria vary depending on the tissue type and location in the body. In any application of a scaffold it is vital to be able to characterise the scaffold before it goes into in vitro testing. In vitro testing allows the cell response to be investigated before its in vivo performance is assessed. A full characterisation of events in vitro and in vivo, in three dimensions (3D), is necessary if a scaffold’s performance and effectiveness is to be fully quantified. This paper focuses on porous scaffolds for bone regeneration, suggests appropriate design criteria for a bone regenerating scaffold and then reviews techniques for obtaining the vitally important quantification of its pore structure. The techniques discussed will include newly developed methods of quantifying X-ray microtomography (μCT) images in 3D and for predicting the scaffolds mechanical properties and the likely paths of fluid flow (and hence potential cell migration). The complications in investigating scaffold performance in vitro are then discussed. Finally, the use of μCT for imaging scaffolds for in vivo tests is reviewed.




Similar content being viewed by others
References
R. Langer, J.P. Vacanti, Science 260, 920–926 (1993). doi:10.1126/science.8493529
E. Lavik, R. Langer, Appl. Microbiol. Biotechnol. 65, 1–8 (2004). doi:10.1007/s00253-004-1580-z
H. Ohgushi, A.I. Caplan, J. Biomed. Mater. Res. 48, 913–927 (1999). doi:10.1002/(SICI)1097-4636(1999)48:6<913::AID-JBM22>3.0.CO;2-0
T. Takezawa, Biomaterials 24, 2267–2275 (2003). doi:10.1016/S0142-9612(03)00038-3
J.R. Jones, L.M. Ehrenfried, L.L. Hench, Biomaterials 27, 964–973 (2006). doi:10.1016/j.biomaterials.2005.07.017
S.F. Hulbert, S.J. Morrison, J.J. Klawitte, J. Biomed. Mater. Res. 6, 347–374 (1972). doi:10.1002/jbm.820060505
L.L. Hench, J.M. Polak, Science 295, 1014–1017 (2002). doi:10.1126/science.1067404
L.J. Gibson, M.F. Ashby, Cellular Solids Structure and Properties (Pergamon Press, Oxford, 1988)
S. Lowell, J.E. Shields, Powder Technol. 38, 121–124 (1984). doi:10.1016/0032-5910(84)80041-8
A.C. Kak, M. Slaney, Principles of Computerized Tomographic Imaging (IEEE Press, London, 1987)
I.D. Xynos, A.J. Edgar, L.D.K. Buttery, L.L. Hench, J.M. Polak, J. Biomed. Mater. Res. 55, 151–157 (2001). doi:10.1002/1097-4636(200105)55:2<151::AID-JBM1001>3.0.CO;2-D
L.L. Hench, R.J. Splinter, W.C. Allen, T.K. Greenlee, J. Biomed. Mater. Res. 5, 117–141 (1971). doi:10.1002/jbm.820050611
P. Sepulveda, J.R. Jones, L.L. Hench, J. Biomed. Mater. Res. 59, 340–348 (2002). doi:10.1002/jbm.1250
J.R. Jones, G. Poologasundarampillai, R.C. Atwood, D. Bernard, P.D. Lee, Biomaterials 28(7), 1404–1413 (2007)
Y. Hiu-Yan, Q. Ling, L. Kwong-Man, Z. Ming, L. Kwok-Sui, C.J. Chun-yiu, J. Biomed. Mater. Res. B Appl. Biomater. 75, 234–242 (2005). doi:10.1002/jbm.b.30240
A.S.P. Lin, T.H. Barrows, S.H. Cartmell, R.E. Guldberg, Biomaterials 24, 481–489 (2003). doi:10.1016/S0142-9612(02)00361-7
S. Cartmell, K. Huynh, A. Lin, S. Nagaraja, R. Guldberg, J. Biomed. Mater. Res. A 69A, 97–104 (2004). doi:10.1002/jbm.a.20118
S.T. Ho, D.W. Hutmacher, Biomaterials 27, 1362–1376 (2006). doi:10.1016/j.biomaterials.2005.08.035
R. Al-Raoush, K.A. Alshibli, Physica A 361, 441–456 (2006). doi:10.1016/j.physa.2005.05.043
B.D. Porter, A.S.P. Lin, A. Peister, D. Hutmacher, R.E. Guldberg, Biomaterials 28, 2525–2533 (2007). doi:10.1016/j.biomaterials.2007.01.013
M.J. Moore, E. Jabbari, E.L. Ritman, L.C. Lu, B.L. Currier, A.J. Windebank et al., J. Biomed. Mater. Res. A 71A, 258–267 (2004). doi:10.1002/jbm.a.30138
B. Otsuki, M. Takemoto, S. Fujibayashi, M. Neo, T. Kokubo, T. Nakamura, Biomaterials 27, 5871–5966 (2006). doi:10.1016/j.biomaterials.2006.08.013
D. Stauffer, Percolation Theory (Taylor and Francis, London, 1985)
R.C. Atwood, J.R. Jones, P.D. Lee, L.L. Hench, Scr. Mater. 51, 1029–1033 (2004). doi:10.1016/j.scriptamat.2004.08.014
A.P. Mangan, R.T. Whitaker, IEEE Trans. Vis. Comput. Graph. 5, 308–321 (1999). doi:10.1109/2945.817348
R. Singh, P.D. Lee, T.C. Lindley, R.J. Dashwood, E. Ferrie, T. Imwinkelried, Acta Biomater. (2008). doi:10.1016/j.actbio.2008.06.014
S.V.N. Jaecques, H. Van Oosterwyck, L. Muraru, T. Van Cleynenbreugel, E. De Smet, M. Wevers et al., Biomaterials 25, 1683–1696 (2004). doi:10.1016/S0142-9612(03)00516-7
D. Lacroix, A. Chateau, M.P. Ginebra, J.A. Planell, Biomaterials 27, 5326–5334 (2006). doi:10.1016/j.biomaterials.2006.06.009
J.R. Jones, P.D. Lee, L.L. Hench, Philos. Trans. R. Soc. Lond. A 364, 263–281 (2006). doi:10.1098/rsta.2005.1689
J.R. Jones, Mater. Today 9, 34–43 (2006). doi:10.1016/S1369-7021(06)71741-2
J.R. Jones, O. Tsigkou, E.E. Coates, M.M. Stevens, J.M. Polak, L.L. Hench, Biomaterials 28, 1653–1663 (2007). doi:10.1016/j.biomaterials.2006.11.022
H. Hagenmueller, S. Hofmann, T. Kohler, H.P. Merkle, D.L. Kaplan, G. Vunjak-Novakovic et al., Ann. Biomed. Eng. 35, 1657–1667 (2007). doi:10.1007/s10439-007-9338-2
T. Hara, E. Tanck, J. Homminga, R. Huiskes, Bone 31, 107–109 (2002). doi:10.1016/S8756-3282(02)00782-2
A. Hilldore, A. Wojtowicz, A.W. Johnson, J. Biomed. Mater. Res. A 82A, 1012–1021 (2007). doi:10.1002/jbm.a.31264
A.C. Jones, C.H. Arns, A.P. Sheppard, D.W. Hutmacher, B.K. Milthorpe, M.A. Knackstedt, Biomaterials 28, 2491–2504 (2007). doi:10.1016/j.biomaterials.2007.01.046
S. Grampp, D. Felsemberg, G. Furhmann, U. Gross, K.J. Wolff, E.F.G. Ring (eds.), Current Research in Ostaeoporosis and Bone Mineral Measurement II (British Institute of Radiology, London, 1992)
R. Mosheiff, B.Y. Klein, I. Leichter, G. Chaimsky, A. Nyska, A. Peyser et al., Biomaterials 13, 462–466 (1992). doi:10.1016/0142-9612(92)90167-M
C. Kirker-Head, V. Karageorgiou, S. Hofmann, R. Fajardo, O. Betz, H.P. Merkle, M. Hilbe, B. von Rechenberg, J. McCool, L. Abrahamsen, A. Nazarian, E. Cory, M. Curtis, D. Kaplan, L. Meinel, Bone 41, 247–255 (2007)
E. Toyota, K. Fujimoto, Y. Ogasawara, T. Kajita, F. Shigeto, T. Matsumoto, M. Goto, F. Kajiya, Circulation 105, 621–626 (2002)
F. Plouraboue, P. Cloetens, C. Fonta, A. Steyer, F. Lauwers, J.P. Marc-Vergnes, J. Microsc.-Oxf. 215, 139–148 (2004)
S. Heinzer, T. Krucker, M. Stampanoni, R. Abela, E.P. Meyer, A. Schuler, P. Schneider, R. Muller, Neuroimage 32, 626–636 (2006)
S. Heinzer, G. Kuhn, T. Krucker, E. Meyer, A. Ulmann-Schuler, M. Stampanoni, M. Gassmann, H.H. Marti, R. Muller, J. Vogel, Neuroimage 39, 1549–1558 (2008)
T. Miclau, C. Lu, Z. Thompson, P. Choi, C. Puttlitz, R. Marcucio, J.A. Helms, J. Orthop. Res. 25, 1552–1558 (2007)
X.P. Zhang, C. Xie, A.S.P. Lin, H. Ito, H. Awad, J.R. Lieberman, P.T. Rubery, E.M. Schwarz, R.J. O’Keefe, R.E. Guldberg, J. Bone. Miner. Res. 20, 2124–2137 (2005)
C.L. Duvall, W.R. Taylor, D. Weiss, R.E. Guldberg, Am. J. Physiol. Heart Circ. Physiol. 287, H302–H310 (2004)
B.J.R.F. Bolland, J.M. Kanczler, D.G. Dunlop, R.O.C. Oreffo, Bone 43, 195–202 (2008)
P. Tafforeau, R. Boistel, E. Boller, A. Bravin, M. Brunet, Y. Chaimanee, P. Cloetens, M. Feist, J. Hoszowska, J.J. Jaeger, R.F. Kay, V. Lazzari, L. Marivaux, A. Nel, C. Nemoz, X. Thibault, P. Vignaud, S. Zabler, Appl. Phys. A 83, 195–202 (2006)
P. Weiss, L. Obadia, D. Magne, X. Bourges, C. Rau, T. Weitkamp, I. Khairoun, J.M. Bouler, D. Chappard, O. Gauthier, G. Daculsi, Biomaterials 24, 4591–4601 (2003)
Acknowledgements
Julian Jones is a Royal Academy of Engineering/EPSRC Research Fellow. The authors gratefully acknowledge financial support for their μCT facility from the Engineering and Physical Sciences Research Council (EP/T26344).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, J.R., Atwood, R.C., Poologasundarampillai, G. et al. Quantifying the 3D macrostructure of tissue scaffolds. J Mater Sci: Mater Med 20, 463–471 (2009). https://doi.org/10.1007/s10856-008-3597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3597-9