Abstract
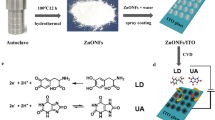
In this paper, graphene foam and ZnO nanorods were prepared by chemical vapor deposition (CVD) and hydrothermal processes, respectively. The hybrids of graphene nanoslices and ZnO nanorods were then fabricated by ultrasonic process. The as-prepared hybrids were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) and Raman spectroscopy. The hybrids possess a slice-like structure with a smooth surface and rod structure, and the lengths of graphene nanoslices are about 3–4 µm. XRD results demonstrated the products are pure without impurities. Raman spectra showed that graphene has a few layers and defect free. The electrical performances of hybrids/ITO electrodes were investigated by cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The sensitivities of the electrode for LD detection are 0.19 and 0.20 µA µM−1 under the interruption of with UA and without UA. The results show that the electrode has good selectivity, reproducibility and stability which would be potential for use in future clinical research.









Similar content being viewed by others
References
W.C. Koller, M.G. Rueda, Mechanism of action of dopaminergic agents in Parkinson’s disease. Neurology 50(6), S11–S14 (1998)
R. Katzenschlager, A.J. Lees, Treatment of Parkinson’s disease: levodopa as the first choice. J Neurol 249(Suppl 2), ii19–ii24 (2002)
S.P. Khor, A. Hsu, The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinson’s disease. Curr Clin Pharmacol 2(3), 234–243 (2007)
R.A. Hawkins, A. Mokashi, I.A. Simpson, An active transport system in the blood–brain barrier may reduce levodopa availability. Exp. Neurol. 195(1), 267–271 (2005)
E.J. Greenhow, L.E. Spencer, Ionic polymerisation as a means of end-point indication in non-aqueous thermometric titrimetry. Part IV. The Determination of catecholamines. Analyst 98(1168), 485–492 (1973)
J. Coello, S. Maspoch, N. Villegas, Simultaneous kinetic-spectrophotometric determination of levodopa and benserazide by Bi- and three-way partial least squares calibration. Talanta 53(3), 627–637 (2000)
M. Karimi, J.L. Carl, S. Loftin et al., Modified high-performance liquid chromatography with electrochemical detection method for plasma measurement of levodopa, 3-O-methyldopa, dopamine, carbidopa and 3,4-dihydroxyphenyl acetic acid. J. Chromatogr. B, 836(1–2), 120–123
J. Wang, Y. Zhou, J. Liang et al., Determination of levodopa and benserazide hydrochloride in pharmaceutical formulations by CZE with amperometric detection. Chromatographia 61(5–6), 265–270 (2005)
N.F. Atta, A. Galal, S.M. Azab, Electrochemical determination of neurotransmitters using gold nanoparticles on nafion/carbon paste modified electrode. J. Electrochem. Soc. 159(10), H765–H771 (2012)
E. Molaakbari, A. Mostafavi, H. Beitollahi et al., Synthesis of ZnO nanorods and their application in the construction of a nanostructure-based electrochemical sensor for determination of levodopa in the presence of carbidopa. Analyst 139(17), 4356–4364 (2014)
C. Sumathi, P. Muthukumaran, S. Radhakrishnan et al., Controlled growth of single-crystalline nanostructured dendrites of α-Fe2O3 blended with MWCNT: a systematic investigation of highly selective determination of L-Dopa. RSC Adv. 4(44), 23050–23057 (2014)
M.J. Cherukara, K. Sasikumar, W. Cha et al., Ultra-fast three-dimensional X-ray imaging of deformation modes in ZnO nanocrystals. Nano Lett. 17, 1102–1108 (2017)
N. Lu, H. Guo, W. Hu et al., Effects of line defects on the electronic properties of ZnO nanoribbons and sheets. J. Mater. Chem. C 5(12), 3121–3129 (2017)
A. Fioravanti, A. Bonanno, M. Mazzocchi et al., Enhanced gas sensing properties of different ZnO 3D hierarchical structures. In Advances in Science and Technology. Trans Tech Publications 99, 48–53 (2017)
P. Sathe, M.T.Z. Myint, S. Dobretsov et al., Removal and regrowth inhibition of microalgae using visible light photocatalysis with ZnO nanorods: a green technology. Sep. Purif. Technol. 162, 61–67 (2016)
F. Xu, L. Sun, Solution-derived ZnO nanostructures for photoanodes of dye-sensitized solar cells. Energy Environ. Sci. 4(3), 818–841 (2011)
J. Yi, J.M. Lee, W.I. Park, Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B 155(1), 264–269 (2011)
S. Lim, D.S. Um, M. Ha, Q. Zhang, Y. Lee, Y. Lin, H. Ko, Broadband omnidirectional light detection in flexible and hierarchical ZnO/Si heterojunction photodiodes. Nano Res. 10, 22–36 (2017)
J.E. Jaffe, J.A. Snyder, Z. Lin et al., LDA and GGA calculations for high-pressure phase transitions in ZnO and MgO. Phys. Rev. B 62(3), 1660 (2000)
A. Shekhawat, R.O. Ritchie, Toughness and strength of nanocrystalline graphene. Nat. Commun. 7, 10546 (2016)
Y. Zhang, S. Gong, Q. Zhang et al., Graphene-based artificial nacre nanocomposites. Chem. Soc. Rev. 45(9), 2378–2395 (2016)
Y. Li, J. Wang, X. Tian et al., Carbon doped molybdenum disulfide nanosheets stabilized on graphene for the hydrogen evolution reaction with high electrocatalytic ability. Nanoscale 8(3), 1676–1683 (2016)
C.S. Boland, S. Barwich, U. Khan et al., High stiffness nano-composite fibres from polyvinylalcohol filled with graphene and boron nitride. Carbon 99, 280–288 (2016)
Ü Özgür, Y.I. Alivov, C. Liu et al., A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98(4), 11 (2005)
T.Y. Perry, S. Shreyas, C. Manish et al., Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 115(7), 2483–2531 (2015)
D.P. Dubal, H. Rudolf, G. Pedro, Development of hybrid materials based on sponge supported reduced graphene oxide and transition metal hydroxides for hybrid energy storage devices. Sci. Rep. 4(1), 7349–7349 (2015)
F. Wang, K. Zhang, Reduced graphene oxide–TiO2 nanocomposite with high photocatalystic activity for the degradation of rhodamine B. J. Mol. Catal. A 345(1–2), 101–107 (2011)
H. Wang, Y. Yang, Y. Liang et al., Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 11(7), 2644–2647 (2011)
M. Shankla, A. Aksimentiev, Modulation of molecular flux using a graphene nanopore capacitor. J. Phys. Chem. B 121, 3724–3733 (2016)
V. Georgakilas, J.N. Tiwari, K.C. Kemp et al., Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev 116(9), 5464–5519 (2016)
Q. Wang, H. Yue, J. Zhang et al., Electrochemical determination of uric acid in the presence of ascorbic acid by hybrid of ZnO nanorods and graphene nanosheets. Ionics. https://doi.org/10.1007/s11581-017-2379-0 (2017)
C.L. Sun, H.H. Lee, J.M. Yang et al., The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 26(8), 3450–3455 (2011)
S. Hou, M.L. Kasner, S. Su et al., Highly sensitive and selective dopamine biosensor fabricated with silanized graphene. J. Phys. Chem. C 114, 14915–14921 (2010)
A. Afkhami, F. Kafrashi, T. Madrakian, Electrochemical determination of levodopa in the presence of ascorbic acid by polyglycine/ZnO nanoparticles/multi-walled carbon nanotubes-modified carbon paste electrode. Ionics 21(10), 2937–2947 (2015)
M. Arvand, N.A. Ghodsi, Voltammetric sensor based on graphene-modified electrode for the determination of trace amounts of L-Dopa in mouse brain extract and pharmaceuticals. J. Solid State Electrochem. 17(3), 775–784 (2013)
M. Arvand, N. Ghodsi, Electrospun TiO2 nanofiber/graphite oxide modified electrode for electrochemical detection of L-Dopa in human cerebrospinal fluid. Sens. Actuators B 204(12), 393–401 (2014)
P. Kanchana, S. Radhakrishnan, M. Navaneethan et al., Electrochemical sensor based on Fe doped hydroxyapatite-carbon nanotubes composite for L-Dopa detection in the presence of uric acid. J. Nanosci. Nanotechnol. 16, 6185–6192 (2016)
M.A. Sheikh-Mohseni, S. Pirsa, Nanostructured conducting polymer/copper oxide as a modifier for fabrication of L-Dopa and uric acid electrochemical sensor. Electroanalysis 28, 1–7 (2016)
Acknowledgements
This work is supported by the Doctoral Scientific Research Fund of Heilongjiang Institute of Technology (2014BJ14) and the Student innovation and entrepreneurship training program of Heilongjiang Province (201611802114).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, Q., Sun, Z. et al. Fabrication the hybrization of ZnO nanorods–Graphene nanoslices and their electrochemical properties to Levodopa in the presence of uric acid. J Mater Sci: Mater Electron 29, 16894–16902 (2018). https://doi.org/10.1007/s10854-018-9784-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9784-7