Abstract
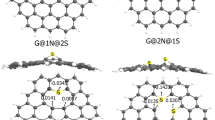
In this work, using density functional theory, we investigate the adsorption process of toxic metal ions (Hg2+, Cd2+, and Pb2+) on graphene nanoflakes (GNF) comprised of various sized oxygen-passivated nanopores to gain molecular insights into the ability of such surfaces in effectively removing the metal ions from contaminated environments. Thermodynamically, the adsorption of these ions on the oxygen-passivated nanopores is shown to be more energetically favorable than that on the pristine surface. The dispersion corrected adsorption energy calculations indicate Hg2+ ion to have the highest and Pb2+ ion the lowest affinities for interaction with such surfaces (Hg2+ > Cd2+ > Pb2+). The highest adsorption of Hg2+ and Cd2+ ions on the surfaces is seen in the 12-crown-3…Hg2+ (− 382.9 kcal/mol) and 12-crown-3…Cd2+ (− 314.7 kcal/mol) complexes, while Pb2+ ion shows the highest adsorption energy for the 18-crown-6 surface (− 195.0 kcal/mol). Noncovalent interaction plots, Hirshfeld charge analysis and energy decomposition analysis conclude that the role of charge transfer in formation of surface…ion complexes is more important than noncovalent interactions and therefore plays a key role in the adsorption of these ions on the surfaces. The magnitude of charge transfer in the complexes follows the order: surface…Hg2+ > surface…Cd2+ > surface…Pb2+, which is consistent with the adsorption energetic order of these metal ions on the surfaces. We also found that Pb2+ ion is mainly adsorbed on the surfaces via electrostatic interactions, while Hg2+ and Cd2+ ions are adsorbed through van der Waals (vdW) interactions. This could be attributed to the presence of vacant p orbitals on Pb2+ ion, which interact with the π bonds on the GNF and oxygen atoms in the nanopores through electrostatic interactions. No such vacant orbitals are available for Hg2+ and Cd2+ ions thus resulting in such ions adsorbing via vdW interactions. The contribution of ΔEorb, induction energy component of total energy, for the surface…Hg2+ complexes is more than that for the surface…Cd2+ and surface…Pb2+ complexes, following the order: surface…Hg2+ > surface…Cd2+ > surface…Pb2+ again, consistent with the calculated adsorption energy order suggesting that charge transfer indeed plays a major role in formation of such surface…ion complexes. Finally, time-dependent density functional theory calculations show that the absorption spectra of the surfaces undergo significant changes, including peak shifts, peak quenching and appearance of new peaks, upon interaction with Hg2+, Cd2+, and Pb2+ ions, such changes being consistent with binding energetics rank order of ions on these surfaces. These complexes would have potential applications in near-infra-red photonics in addition to their service of effectively remediating of toxic heavy elements from contaminated environments.
Graphic abstract









Similar content being viewed by others
References
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43(3):246–253. https://doi.org/10.4103/0253-7613.81505
https://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/
Novoselov KS, Jiang D, Schedin F, Booth T, Khotkevich V, Morozov S, Geim AK (2005) Two-dimensional atomic crystals. Proc Natl Acad Sci 102(30):10451–10453. https://doi.org/10.1073/pnas.0502848102
Xu J, Cao Z, Zhang Y, Yuan Z, Lou Z, Xu X, Wang X (2018) A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: preparation, application, and mechanism. Chemosphere 195:351–364. https://doi.org/10.1016/j.chemosphere.2017.12.061
Sherlala AIA, Raman AAA, Bello MM, Asghar A (2018) A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 193:1004–1017. https://doi.org/10.1016/j.chemosphere.2017.11.093
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504. https://doi.org/10.1016/j.molliq.2017.01.064
Yang Z-y, Dai N-n, Lu R-t, Huang Z-h, Kang F-y (2016) A review of graphene composite-based sensors for detection of heavy metals. Carbon 100(104):260–262. https://doi.org/10.1016/j.carbon.2015.12.055
Chang J, Zhou G, Christensen ER, Heideman R, Chen J (2014) Graphene-based sensors for detection of heavy metals in water: a review. Anal Bioanal Chem 406(16):3957–3975. https://doi.org/10.1007/s00216-014-7804-x
Abu-Nada A, McKay G, Abdala A (2020) Recent advances in applications of hybrid graphene materials for metals removal from wastewater. Nanomaterials 10(3):595–627. https://doi.org/10.3390/nano10030595
Kommu A, Singh JK (2020) A review on graphene-based materials for removal of toxic pollutants from wastewater. Soft Mater. https://doi.org/10.1080/1539445X.2020.1739710
Guerrero-Fajardo CA, Giraldo L, Moreno-Piraján JC (2020) Preparation and characterization of graphene oxide for Pb(II) and Zn(II) ions adsorption from aqueous solution: experimental, thermodynamic and kinetic study. Nanomaterials 10(6):1022–1050. https://doi.org/10.3390/nano10061022
Kong Q, Preis S, Li L, Luo P, Wei C, Li Z, Hu Y, Wei C (2020) Relations between metal ion characteristics and adsorption performance of graphene oxide: a comprehensive experimental and theoretical study. Sep Purif Technol 232:115956. https://doi.org/10.1016/j.seppur.2019.115956
El-Fawal EM, Saad L, Moustafa YM (2020) Computational DFT study of magnetite/graphene oxide nanoadsorbent: interfacial chemical behavior and remediation performance of heavy metal hydrates from aqueous system. Water Environ Res. https://doi.org/10.1002/wer.1325
Berber MR (2020) Current advances of polymer composites for water treatment and desalination. J Chem 2020:1–18. https://doi.org/10.1155/2020/7608423
Li L, Zhao L, Ma J, Tian Y (2020) Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions. Green Process Synth 9(1):294–303. https://doi.org/10.1515/gps-2020-0030
Lee J, Yang Z, Zhou W, Pennycook SJ, Pantelides ST, Chisholm MF (2014) Stabilization of graphene nanopore. Proc Natl Acad Sci 111(21):7522–7526. https://doi.org/10.1073/pnas.1400767111
Xiao J, Mei D, Li X, Xu W, Wang D, Graff GL, Bennett WD, Nie Z, Saraf LV, Aksay IA, Liu J, Zhang J-G (2011) Hierarchically porous graphene as a lithium-air battery electrode. Nano Lett 11(11):5071–5078. https://doi.org/10.1021/nl203332e
Datta D, Li J, Shenoy VB (2014) Defective graphene as a high-capacity anode material for Na- and Ca-ion batteries. ACS Appl Mater Interfaces 6(3):1788–1795. https://doi.org/10.1021/am404788e
O’Hern SC, Boutilier MSH, Idrobo J-C, Song Y, Kong J, Laoui T, Atieh M, Karnik R (2014) Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett 14(3):1234–1241. https://doi.org/10.1021/nl404118f
Rollings RC, Kuan AT, Golovchenko JA (2016) Ion selectivity of graphene nanopores. Nat Commun 7:11408–11414. https://doi.org/10.1038/ncomms11408
Surwade SP, Smirnov SN, Vlassiouk IV, Unocic RR, Veith GM, Dai S, Mahurin SM (2015) Water desalination using nanoporous single-layer graphene. Nat Nanotechnol 10(5):459–464. https://doi.org/10.1038/nnano.2015.37
Bell DC, Lemme MC, Stern LA, Williams JR, Marcus CM (2009) Precision cutting and patterning of graphene with helium ions. Nanotechnology 20(45):455301–455307. https://doi.org/10.1088/0957-4484/20/45/455301
Bieri M, Treier M, Cai J, Aït-Mansour K, Ruffieux P, Gröning O, Gröning P, Kastler M, Rieger R, Feng X, Müllen K, Fasel R (2009) Porous graphenes: two-dimensional polymer synthesis with atomic precision. ChemComm 45:6919–6921. https://doi.org/10.1039/b915190g
Kim M, Safron NS, Han E, Arnold MS, Gopalan P (2010) Fabrication and characterization of large-area, semiconducting nanoperforated graphene materials. Nano Lett 10(4):1125–1131. https://doi.org/10.1021/nl9032318
Russo CJ, Golovchenko JA (2012) Atom-by-atom nucleation and growth of graphene nanopores. Proc Natl Acad Sci 109(16):5953–5957
Tsetseris L, Pantelides ST (2009) Adatom complexes and self-healing mechanisms on graphene and single-wall carbon nanotubes. Carbon 47(3):901–908. https://doi.org/10.1016/j.carbon.2008.12.002
Zan R, Ramasse QM, Bangert U, Novoselov KS (2012) Graphene reknits its holes. Nano Lett 12(8):3936–3940. https://doi.org/10.1021/nl300985q
Guo J, Lee J, Contescu CI, Gallego NC, Pantelides ST, Pennycook SJ, Moyer BA, Chisholm MF (2014) Crown ethers in graphene. Nat Commun 5(1):1–6. https://doi.org/10.1038/ncomms6389
Maarouf AA, Nistor RA, Afzali-Ardakani A, Kuroda MA, Newns DM, Martyna GJ (2013) Crown graphene nanomeshes: highly stable chelation-doped semiconducting materials. J Chem Theory Comput 9(5):2398–2403. https://doi.org/10.1021/ct4000636
Heath JJ, Kuroda MA (2018) First principles studies of the interactions between alkali metal elements and oxygen-passivated nanopores in graphene. Phys Chem Chem Phys 20(40):25822–25828. https://doi.org/10.1039/C8CP04958K
Olsson E, Chai G, Dove M, Cai Q (2019) Adsorption and migration of alkali metals (Li, Na, and K) on pristine and defective graphene surfaces. Nanoscale 11(12):5274–5284. https://doi.org/10.1039/C8NR10383F
Azamat J (2016) Functionalized graphene nanosheet as a membrane for water desalination using applied electric fields: insights from molecular dynamics simulations. J Phys Chem C 120(41):23883–23891. https://doi.org/10.1021/acs.jpcc.6b08481
Cohen-Tanugi D, Grossman JC (2012) Water desalination across nanoporous graphene. Nano Lett 12(7):3602–3608. https://doi.org/10.1021/nl3012853
Sint K, Wang B, Král P (2008) Selective ion passage through functionalized graphene nanopores. J Am Chem Soc 130(49):16448–16449. https://doi.org/10.1021/ja804409f
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1):51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Frisch M, Trucks G, Schlegel H et al (2009) Gaussian 09, Revision D. 01. Gaussian, Wallingford
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566. https://doi.org/10.1080/00268977000101561
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104–154123. https://doi.org/10.1063/1.3382344
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44(2):129–138. https://doi.org/10.1007/BF00549096
Lu T, Chen F (2012) Multiwfn: a multifunctional wave-function analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Runge E, Gross EKU (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52(12):997–1000. https://doi.org/10.1103/PhysRevLett.52.997
Raghi KR, Sherin DR, Saumya MJ, Arun PS, Sobha VN, Manojkumar TK (2018) Computational study of molecular electrostatic potential, docking and dynamics simulations of gallic acid derivatives as ABL inhibitors. Comput Biol Chem 74:239–246. https://doi.org/10.1016/j.compbiolchem.2018.04.001
Shtepliuk I, Yakimova R (2018) Interband transitions in closed-shell vacancy containing graphene quantum dots complexed with heavy metals. Phys Chem Chem Phys 20(33):21528–21543. https://doi.org/10.1039/C8CP03306D
Shtepliuk I, Caffrey NM, Iakimov T, Khranovskyy V, Abrikosov IA, Yakimova R (2017) On the interaction of toxic heavy metals (Cd, Hg, Pb) with graphene quantum dots and infinite graphene. Sci Rep 7(1):1–17. https://doi.org/10.1038/s41598-017-04339-8
Ghenaatian HR, Shakourian-Fard M, Kamath G (2019) The effect of sulfur and nitrogen/sulfur co-doping in graphene surface on the adsorption of toxic heavy metals (Cd, Hg, Pb). J Mater Sci 54(20):13175–13189. https://doi.org/10.1007/s10853-019-03791-3
Ghenaatian HR, Shakourian-Fard M, Moghadam MR, Kamath G, Rahmanian M (2019) Tailoring of graphene quantum dots for toxic heavy metals detection. Appl Phys A 125(11):754–765. https://doi.org/10.1007/s00339-019-3042-6
Thompho S, Rungrotmongkol T, Saengsawang O, Hannongbua S (2017) A computational study of adsorption of divalent metal ions on graphene oxide. Songklanakarin J Sci Technol 39(6):773–778. https://doi.org/10.14456/sjst-psu.2017.94
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506. https://doi.org/10.1021/ja100936w
Cao S, Wang J, Ding Y, Sun M, Ma F (2017) Visualization of weak interactions between quantum dot and graphene in hybrid materials. Sci Rep 7(1):417–424. https://doi.org/10.1038/s41598-017-00542-9
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J Chem Soc Faraday Trans 87(18):2995–2999. https://doi.org/10.1039/FT9918702995
Fonseca Guerra C, Wijst T, Poater J, Swart M, Bickelhaupt FM (2010) Adenine versus guanine quartets in aqueous solution: dispersion-corrected DFT study on the differences in π-stacking and hydrogen-bonding behavior. Theor Chem Acc 125:245–252. https://doi.org/10.1007/s00214-009-0634-9
Silva AM, Pires MS, Freire VN, Albuquerque EL, Azevedo DL, Caetano EWS (2010) Graphene nanoflakes: thermal stability, infrared signatures, and potential applications in the field of spintronics and optical nanodevices. J Phys Chem C 114(41):17472–17485. https://doi.org/10.1021/jp105728p
Dahal R, Lin JY, Jiang HX, Zavada JM (2011) Near infrared photonic devices based on Er-doped GaN and InGaN. Opt Mater 33(7):1066–1070. https://doi.org/10.1016/j.optmat.2010.10.002
Wang Z, Li H, Luo M, Chen T, Xia X, Chen H, Ma C, Guo J, He Z, Song Y, Liu J, Jiang X, Zhang H (2020) MXene photonic devices for near-infrared to mid-infrared ultrashort pulse generation. ACS Appl Nano Mater 3(4):3513–3522. https://doi.org/10.1021/acsanm.0c00241
Zhang Y, Ye J, Xu J, Song J, Yao T, Zhou P (2020) Dual-wavelength random distributed feedback fiber laser with wavelength, linewidth, and power ratio tunability. Opt Express 28(7):10515–10523. https://doi.org/10.1364/OE.390796
Türker-Kaya S, Huck CW (2017) A review of mid-infrared and near-infrared imaging: principles, concepts and applications in plant tissue analysis. Molecules 22(1):168–187. https://doi.org/10.3390/molecules22010168
Marris-Morini D, Vakarin V, Ramirez JM, Liu Q, Ballabio A, Frigerio J, Montesinos M, Alonso-Ramos C, Roux XL, Serna S, Benedikovic D, Chrastina D, Vivien L, Isella G (2018) Germanium-based integrated photonics from near- to mid-infrared applications. Nanophotonics 7(11):1781–1793. https://doi.org/10.1515/nanoph-2018-0113
Chang P, Niu W, Qu L, Zhang S (2019) Two-way rewritable and stable photonic patterns enabled by near-infrared laser-responsive shape memory photonic crystals. J Mater Chem C 7:1896–1903. https://doi.org/10.1039/D0TC00863J
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Jahrom University and Birjand University of Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Christopher Blanford.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghenaatian, H.R., Shakourian-Fard, M. & Kamath, G. Adsorption mechanism of toxic heavy metal ions on oxygen-passivated nanopores in graphene nanoflakes. J Mater Sci 55, 15826–15844 (2020). https://doi.org/10.1007/s10853-020-05113-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05113-4