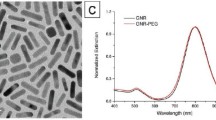
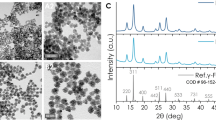
Abstract
Chemotherapy in cancer treatment usually leads to serious side effects on patients due to the unselectiveness and high toxicity on normal cells of cancer drugs. Loading cancer drugs into nano-platforms could be an alternative approach to effectively deliver drugs to tumors and reduce toxic exposure on healthy cells. In this work, we synthesized drug delivery nano-systems based on Fe3O4 nanoparticles (obtained from co-precipitation reaction) which could provide targeting of drugs to the tumor sites by an external magnetic field. Also, the magnetic nanoparticles (MNPs) could generate heat to kill cancer cells at a certain temperature range. The systems were designed for loading anticancer agent doxorubicin by using alginate-coated iron oxide MNPs. It was found that the loading was achieved by complex formation of doxorubicin and the alginate layer. Various concentrations of alginate solutions produced different sizes as well as drug loading capacities of the nanoparticles. The highest loading content of 18.96% achieved at the alginate concentration of 4 mg ml−1, corresponding to the mass ratio of alginate to Fe3O4 of around 1:2. The magnetic properties, especially the inductive heating effect of the nanoparticles, along with the impact of the systems on tumor cells were investigated. The results proved that the nanoparticles can serve as a good drug delivery system, in terms of both effective hyperthermia and chemotherapy.












Similar content being viewed by others
References
Nasongkla N, Bey E, Ren J et al (2006) Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett 6:3–6
Hong G, Yuan R, Liang B et al (2008) Folate-functionalized polymeric micelle as hepatic carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor drugs. Biomed Microdevice 10:693–700
Yu MK, Jeong YY, Park J, et al (2008) Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo**. Evolution:1–5
Ma HL, Xu YF, Qi XR et al (2008) Superparamagnetic iron oxide nanoparticles stabilized by alginate: pharmacokinetics, tissue distribution, and applications in detecting liver cancers. Int J Pharm 354:217–226
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60:1252–1265
Lin MM, Kim DK, El Haj AJ, Dobson J (2008) Development of superparamagnetic iron oxide nanoparticles (SPIONS) for translation to clinical applications. IEEE Trans Nanobiosci 7:298–305
Mahmoudi M, Simchi A, Imani M et al (2008) Optimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imaging. J Phys Chem B 112:14470–14481
Shete PB, Patil RM, Tiwale BM, Pawar SH (2015) Water dispersible oleic acid-coated Fe3O4 nanoparticles for biomedical applications. J Magn Magn Mater 377:406–410
Jia Y, Yuan M, Yuan H et al (2012) Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biodegradable PLGA nanocarriers for intratumoral drug delivery. Int J Nanomed 7:1697–1708
Maeng JH, Lee D-H, Jung KH et al (2010) Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 31:4995–5006
Unsoy G, Khodadust R, Yalcin S et al (2014) Synthesis of doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur J Pharm Sci 62:243–250
Insausti M, Salado J, Castellanos I et al (2012) Tailoring biocompatible Fe3O4 nanoparticles for applications to magnetic hyperthermia. Proc SPIE 8232:823210/1–823210/8
Kikuchi T, Kasuya R, Endo S, Nakamura A (2010) Preparation of magnetite aqueous dispersion for magnetic fluid hyperthermia. J Magn Magn Mater 323:1216–1222
Ang KL, Venkatraman S, Ramanujan RV (2007) Magnetic PNIPA hydrogels for hyperthermia applications in cancer therapy. Mater Sci Eng C 27:347–351
Longhi A, Ferrari S, Bacci G, Specchia S (2007) Long-term follow-up of patients with doxorubicin-induced cardiac toxicity after chemotherapy for osteosarcoma. Anticancer Drugs 18:737–744
Ahn D, Lee J, Park S et al (2014) Doxorubicin-loaded alginate-g-poly(N-isopropylacrylamide) micelles for cancer imaging and therapy. ACS Appl Mater Interfaces 6(24):22069–22077
Cheng Y, Yu S, Zhen X et al (2012) Alginic acid nanoparticles prepared through counterion complexation method as a drug delivery system. ACS Appl Mater Interfaces 4:5325–5332
Javid A, Ahmadian S, Saboury AA et al (2013) Chitosan-coated superparamagnetic iron oxide nanoparticles for doxorubicin delivery: synthesis and anticancer effect against human ovarian cancer cells. Chem Biol Drug Des 82:296–306
Baghbani F, Moztarzadeh F, Mohandesi JA et al (2016) Formulation design, preparation and characterization of multifunctional alginate stabilized nanodroplets. Int J Biol Macromol 89:550–558
Sengupta IS, Shah SH, Shah N (2015) A review on: alginate forming in situ gel for treating peptic ulcers and reflux disorders. J Pharm Sci Biosci Res 5:171–179
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci (Oxford) 37:106–126
Nguyen HN, Hoang TMN, Mai TTT et al (2015) Enhanced cellular uptake and cytotoxicity of folate decorated doxorubicin loaded PLA–TPGS nanoparticles. Adv Nat Sci Nanosci Nanotechnol 6:25005
Nguyen HN, Thi HHT, Le Quang D et al (2012) Apoptosis induced by paclitaxel-loaded copolymer PLA–TPGS in Hep-G2 cells. Adv Nat Sci Nanosci Nanotechnol 3:45005
Phan QT, Le MH, Le TTH et al (2016) Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA–PEG micellar nano systems with various PLA:PEG ratios. Int J Pharm 507:32–40
Devkota J, Mai TTT, Stojak K et al (2014) Synthesis, inductive heating, and magnetoimpedance-based detection of multifunctional Fe3O4 nanoconjugates. Sensors Actuators B Chem 190:715–722
Huong LTT, Nam NH, Doan DH et al (2016) Folate attached, curcumin loaded Fe3O4 nanoparticles: a novel multifunctional drug delivery system for cancer treatment. Mater Chem Phys 172:98–104
Thu HP, Huong LTT, Nhung HTM et al (2011) Fe3O4/o-carboxylmethyl chitosan/curcumin-based nanodrug system for chemotherapy and fluorescence imaging in HT29 cancer cell line. Chem Lett 11:1–4
Nguyen XP, Tran DL, Ha PT et al (2012) Iron oxide-based conjugates for cancer theragnostics. Adv Nat Sci Nanosci Nanotechnol 3:33001
Kallumadil M, Tada M, Nakagawa T et al (2009) Suitability of commercial colloids for magnetic hyperthermia. J Magn Magn Mater 321:1509–1513
Salas G, Veintemillas-Verdaguer S, Morales MDP (2013) Relationship between physico-chemical properties of magnetic fluids and their heating capacity. Int J Hyperth Off J Eur Soc Hyperth Oncol N Am Hyperth Group 29:768–776
Tran LD, Hoang NMT, Mai TT et al (2010) Nanosized magnetofluorescent Fe3O4-curcumin conjugate for multimodal monitoring and drug targeting. Colloids Surf A 371:104–112
Wang G, Su X, Yang S et al (2012) The double-effect mechanism between Fe3O4 nanoparticles and MSA-capped CdTe QDs. J Lumin 132:2505–2511
Liao S-H, Liu C-H, Bishnu Prasad B et al (2015) Functionalized magnetic iron oxide/alginate core–shell nanoparticles for targeting hyperthermia. Int J Nanomed 10:3315–3328
Araújo-Neto RP, Silva-Freitas EL, Carvalho JF et al (2014) Monodisperse sodium oleate coated magnetite high susceptibility nanoparticles for hyperthermia applications. J Magn Magn Mater 364:72–79
Vasconcelos IB, Da Silva TG, Militão GCG et al (2012) Cytotoxicity and slow release of the anti-cancer drug doxorubicin from ZIF-8. RSC Adv 2:9437–9442
Kayal S, Ramanujan RV (2010) Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater Sci Eng C 30:484–490
Nigam S, Barick KC, Bahadur D (2011) Development of citrate-stabilized Fe3O4 nanoparticles: conjugation and release of doxorubicin for therapeutic applications. J Magn Magn Mater 323:237–243
Hervault A, Dunn AE, Lim M et al (2016) Doxorubicin loaded dual pH- and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 8:21–24
Fischer MJE (2010) Amine coupling through EDC/NHS: a practical approach methods. Mol Biol 627:55–73
Neuberger T, Schöpf B, Hofmann H et al (2005) Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater 293:483–496
Gupta A, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021
Hałupka-Bryl M, Bednarowicz M, Dobosz B et al (2015) Doxorubicin loaded PEG-b-poly(4-vinylbenzylphosphonate) coated magnetic iron oxide nanoparticles for targeted drug delivery. J Magn Magn Mater 384:320–327
Marín T, Montoya P, Arnache O, Calderón J (2016) Influence of surface treatment on magnetic properties of Fe3O4 nanoparticles synthesized by electrochemical method. J Phys Chem B 120:6634–6645
Khalkhali M, Sadighian S, Rostamizadeh K et al (2015) Synthesis and characterization of dextran coated magnetite nanoparticles for simultaneous diagnostics and therapy. Bioimpacts 5:141–150
Aliahmad M, Nasiri Moghaddam N (2013) Synthesis of maghemite (γ-Fe2O3) nanoparticles by thermal-decomposition of magnetite (Fe3O4) nanoparticles. Mater Sci Pol 31:264–268
Pereira C, Pereira AM, Fernandes C et al (2012) Superparamagnetic MFe2O4 (M = Fe Co, Mn) nanoparticles: tuning the particle size and magnetic properties through a novel one-step coprecipitation route. Chem Mater 24:1496–1504
Cao S-W, Zhu Y-J, Chang J (2008) Fe3O4 polyhedral nanoparticles with a high magnetization synthesized in mixed solvent ethylene glycol–water system. New J Chem 32:1526–1530
Akbarzadeh A, Mikaeili H, Zarghami N et al (2012) Preparation and in vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomed 7:511–526
Chen F-H, Zhang L-M, Chen Q-T et al (2010) Synthesis of a novel magnetic drug delivery system composed of doxorubicin-conjugated Fe3O4 nanoparticle cores and a PEG-functionalized porous silica shell. Chem Commun (Camb) 46:8633–8635
Lima E, De Biasi E, Mansilla MV et al (2013) Heat generation in agglomerated ferrite nanoparticles in an alternating magnetic field. J Phys D Appl Phys 46:045002
Chin SF, Iyer KS, Saunders M et al (2009) Encapsulation and sustained release of curcumin using superparamagnetic silica reservoirs. Chem Eur J 15:5661–5665
Jordan A, Scholz R, Wust P et al (1999) Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 201:413–419
Johannsen M, Gneveckow U, Thiesen B et al (2007) Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 52:1653–1661
Van Landeghem FKH, Maier-hauff K, Jordan A et al (2009) Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 30:52–57
Maier-hauff K, Ulrich F, Nestler D et al (2011) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103:317–324
Liu XL, Fan HM, Yi JB et al (2012) Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J Mater Chem 22:8235–8244
De La Presa P, Luengo Y, Multigner M et al (2012) Study of heating efficiency as a function of concentration, size, and applied field in g-Fe2O3 nanoparticles. J Phys Chem C 116:25602–25610
Serantes D, Simeonidis K, Angelakeris M et al (2014) Multiplying magnetic hyperthermia response by nanoparticle assembling. J Phys Chem C 118:5927–5934
Nemati Z, Alonso J, Rodrigo I et al (2018) Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J Phys Chem C 122:2367–2381
Piñeiro-Redondo Y, Bañobre-López M, Pardiñas-Blanco I et al (2011) The influence of colloidal parameters on the specific power absorption of PAA-coated magnetite nanoparticles. Nanoscale Res Lett 6:383
Engineer C, Parikh J, Raval A (2011) Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater Artif Organs 25:79–85
Yang F, Zhang X, Song L et al (2015) Controlled drug release and hydrolysis mechanism of polymer-magnetic nanoparticle composite. ACS Appl Mater Interfaces 7:9410–9419
Margaritis A, Manocha B (2010) Controlled release of doxorubicin from doxorubicin/polyglutamic acid ionic complex. J Nanomater 2010:780171
Estrella V, Chen T, Lloyd M et al (2013) Acidity generated by the tumor microenvironment drives local invasion. Can Res 73:1524–1535
Javid A, Ahmadian S, Saboury A, Rezaei-zarchi S (2011) Anticancer effect of doxorubicin loaded heparin based super-paramagnetic iron oxide nanoparticles against the human ovarian cancer cells. Int J Biomed Biol Eng 5:141–145
Prabha G, Raj V (2017) Sodium alginate–polyvinyl alcohol–bovin serum albumin coated Fe3O4nanoparticles as anticancer drug delivery vehicle: doxorubicin loading and in vitro release study and cytotoxicity to HepG2 and L02 cells. Mater Sci Eng C 79:410–422
Ghanbari M, Asadi A (2016) Study of the cytotoxicity effect of doxorubicin-loaded/folic acid-targeted super paramagnetic iron oxide nanoparticles on AGS cancer cell line. J Nanomed Nanotechnol 7:368
Acknowledgements
This work was financially supported by the National Foundation for Science and Technology Development of Vietnam-NAFOSTED under Grant No. 106-YS.06-2015.14 (HPT). The authors would like to thank Dr. Ung Thi Dieu Thuy for her fluorescence measurement and Ms. Cao Phuong Lien for her English proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Rights and permissions
About this article
Cite this article
Le, T.T.H., Bui, T.Q., Ha, T.M.T. et al. Optimizing the alginate coating layer of doxorubicin-loaded iron oxide nanoparticles for cancer hyperthermia and chemotherapy. J Mater Sci 53, 13826–13842 (2018). https://doi.org/10.1007/s10853-018-2574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2574-z