Abstract
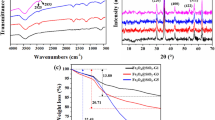
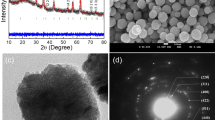
This work reports the synthesis of surface-modified iron oxide magnetic nanotubes (Fe3O4NT) with poly(amido amine) dendrimers of the third generation (PAMAM-G3) as novel nanomaterials for potential drug-delivery applications. Fe3O4NT were obtained by reduction of α-Fe2O3 nanotubes, which were synthesized following a hydrothermal strategy using SO4 2−/H2PO4 − to control the size and morphology of the prepared materials. Fe3O4NT were further functionalized with PAMAM-G3 moieties using a silane coupling agent. Pristine and PAMAM-modified Fe3O4NT were characterized through TEM, FTIR, XRD, N2 adsorption–desorption isotherms and VSM measurements, which confirmed the nanotubular morphology and magnetic behavior for both systems, and TGA analyses, which revealed a PAMAM grafting percentage of 16.8%. The effect of PAMAM conjugation on the adsorption and release properties of Fe3O4NT was examined using silibinin as model poorly soluble drug compound. Our results revealed that PAMAM grafting increased the maximum amount of adsorbed drug from 675 mg g−1 in pristine Fe3O4NT to 825 mg g−1 in PAMAM-Fe3O4NT. These quantities exceed by far the drug-loading capacity of other pristine and PAMAM-modified nanotubular systems, which constitutes a relevant outcome for the present study.








Similar content being viewed by others
References
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83(5):761–769. doi:10.1038/sj.clpt.6100400
Faraji AH, Wipf P (2009) Nanoparticles in cellular drug delivery. Bioorg Med Chem 17(8):2950–2962. doi:10.1016/j.bmc.2009.02.043
Kievit FM, Zhang MQ (2011) Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res 44(10):853–862. doi:10.1021/ar2000277
Yu MK, Park J, Jon S (2012) Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2(1):3–44. doi:10.7150/thno.3463
Liu Z, Sun XM, Nakayama-Ratchford N, Dai HJ (2007) Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 1(1):50–56. doi:10.1021/nn700040t
Goldberg M, Langer R, Jia XQ (2007) Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym E 18(3):241–268. doi:10.1163/156856207779996931
Song YY, Schmidt-Stein F, Bauer S, Schmuki P (2009) Amphiphilic TiO2 nanotube arrays: an actively controllable drug delivery system. J Am Chem Soc 131(12):4230–4232. doi:10.1021/ja810130h
Popat KC, Eltgroth M, La Tempa TJ, Grimes CA, Desai TA (2007) Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 3(11):1878–1881. doi:10.1002/smll.200700412
Li XM, Wang L, Fan YB, Feng QL, Cui FZ (2012) Biocompatibility and toxicity of nanoparticles and nanotubes. J Nanomater 2012:548389. doi:10.1155/2012/548389
Wang QJ, Liu RJ, Shen XQ, Zou LL, Wu DM (2014) Mesoporous iron oxide nanofibers and their loading capacity of curcumin. J Nanosci Nanotechnol 14(4):2871–2877. doi:10.1166/jnn.2014.8624
Fratila RM, Rivera-Fernandez S, de la Fuente JM (2015) Shape matters: synthesis and biomedical applications of high aspect ratio magnetic nanomaterials. Nanoscale 7(18):8233–8260. doi:10.1039/c5nr01100k
Horst MF, Lassalle V, Ferreira ML (2015) Nanosized magnetite in low cost materials for remediation of water polluted with toxic metals, azo- and antraquinonic dyes. Front Environ Sci Eng 9(5):746–769. doi:10.1007/s11783-015-0814-x
Keerthana DS, Namratha K, Byrappa K, Yathirajan HS (2015) Facile one-step fabrication of magnetite particles under mild hydrothermal conditions. J Magn Magn Mater 378:551–557. doi:10.1016/j.jmmm.2014.10.176
Jia CJ, Sun LD, Yan ZG, You LP, Luo F, Han XD, Pang YC, Zhang Z, Yan CH (2005) Iron oxide nanotubes: single-crystalline iron oxide nanotubes. Angew Chem Int Ed 44(28):4328–4333. doi:10.1002/anie.200463038
Jia CJ, Sun LD, Luo F, Han XD, Heyderman LJ, Yan ZG, Yan CH, Zheng K, Zhang Z, Takano M, Hayashi N, Eltschka M, Klaui M, Rudiger U, Kasama T, Cervera-Gontard L, Dunin-Borkowski RE, Tzvetkov G, Raabe J (2008) Large-scale synthesis of single-crystalline iron oxide magnetic nanorings. J Am Chem Soc 130(50):16968–16977. doi:10.1021/ja805152t
Mizutani N, Iwasaki T, Watano S, Yanagida T, Kawai T (2010) Size control of magnetite nanoparticles in hydrothermal synthesis by coexistence of lactate and sulfate ions. Curr Appl Phys 10(3):801–806. doi:10.1016/j.cap.2009.09.018
Yue ZG, Wei W, You ZX, Yang QZ, Yue H, Su ZG, Ma GH (2011) Iron oxide nanotubes for magnetically guided delivery and pH-activated release of insoluble anticancer drugs. Adv Funct Mater 21(18):3446–3453. doi:10.1002/adfm.201100510
Park J, Kadasala NR, Abouelmagd SA, Castanares MA, Collins DS, Wei A, Yeo Y (2016) Polymer-iron oxide composite nanoparticles for EPR-independent drug delivery. Biomaterials 101:285–295. doi:10.1016/j.biomaterials.2016.06.007
Gonzalez-Moragas L, Yu SM, Carenza E, Laromaine A, Roig A (2015) Protective effects of bovine serum albumin on superparamagnetic iron oxide nanoparticles evaluated in the nematode Caenorhabditis elegans. ACS Biomater Sci Eng 1(11):1129–1138. doi:10.1021/acsbiomaterials.5b00253
Castillo PM, de la Mata M, Casula MF, Sanchez-Alcazar JA, Zaderenko AP (2014) PEGylated versus non-PEGylated magnetic nanoparticles as camptothecin delivery system. Beilstein J Nanotechnol 5:1312–1319. doi:10.3762/bjnano.5.144
Mahdavi M, Bin Ahmad M, Haron MJ, Namvar F, Nadi B, Ab Rahman MZ, Amin J (2013) Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 18(7):7533–7548. doi:10.3390/molecules18077533
Tajabadi M, Khosroshahi ME, Bonakdar S (2013) An efficient method of SPION synthesis coated with third generation PAMAM dendrimer. Colloid Surf A 431:18–26. doi:10.1016/j.colsurfa.2013.04.003
Shi X, Wang SH, Swanson SD, Ge S, Cao Z, Van Antwerp ME, Landmark KJ, Baker JR (2008) Dendrimer-functionalized shell-crosslinked iron oxide nanoparticles for in-vivo magnetic resonance imaging of tumors. Adv Mater 20(9):1671–1678. doi:10.1002/adma.200702770
Strable E, Bulte JWM, Moskowitz B, Vivekanandan K, Allen M, Douglas T (2001) Synthesis and characterization of soluble iron oxide–dendrimer composites. Chem Mater 13(6):2201–2209. doi:10.1021/cm010125i
Deriu MA, Popescu LM, Ottaviani MF, Danani A, Piticescu RM (2016) Iron oxide/PAMAM nanostructured hybrids: combined computational and experimental studies. J Mater Sci 51(4):1996–2007. doi:10.1007/s10853-015-9509-8
Campos CH, Diaz CF, Guzman JL, Alderete JB, Torres CC, Jimenez VA (2016) PAMAM-conjugated alumina nanotubes as novel noncytotoxic nanocarriers with enhanced drug loading and releasing performances. Macromol Chem Phys 217(15):1712–1722. doi:10.1002/macp.201600136
Torres CC, Campos CH, Diaz C, Jimenez VA, Vidal F, Guzman L, Alderete JB (2016) PAMAM-grafted TiO2 nanotubes as novel versatile materials for drug delivery applications. Mater Sci Eng C J 65:164–171. doi:10.1016/j.msec.2016.03.104
Devarakonda B, Hill RA, de Villiers MM (2004) The effect of PAMAM dendrimer generation size and surface functional group on the aqueous solubility of nifedipine. Int J Pharm 284(1–2):133–140. doi:10.1016/j.ijpharm.2004.07.006
Barra PA, Barraza L, Jimenez VA, Gavin JA, Alderete JB (2014) Complexation of mefenamic acid by low-generation PAMAM dendrimers: insight from NMR spectroscopy studies and molecular dynamics simulations. Macromol Chem Phys 215(4):372–383. doi:10.1002/macp.201300398
Fant K, Esbjorner EK, Jenkins A, Grossel MC, Lincoln P, Norden B (2010) Effects of PEGylation and acetylation of PAMAM dendrimers on DNA binding, cytotoxicity and in vitro transfection efficiency. Mol Pharm 7(5):1734–1746. doi:10.1021/mp1001312
Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H (2007) Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjugate Chem 18(6):2054–2060. doi:10.1021/bc0603889
Markatou E, Gionis V, Chryssikos GD, Hatziantoniou S, Georgopoulos A, Demetzos C (2007) Molecular interactions between dimethoxycurcumin and Pamam dendrimer carriers. Int J Pharm 339(1–2):231–236. doi:10.1016/j.ijpharm.2007.02.037
Borowska K, Wolowiec S, Rubaj A, Glowniak K, Sieniawska E, Radej S (2012) Effect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene-in vivo study. Int J Pharm 426(1–2):280–283. doi:10.1016/j.ijpharm.2012.01.041
Savic AB, Cokesa D, Lazarevic S, Jokic B, Janackovic D, Petrovic R, Zivkovic LS (2016) Tailoring of magnetite powder properties for enhanced phosphate removal: effect of PEG addition in the synthesis process. Powder Technol 301:511–519. doi:10.1016/j.powtec.2016.06.028
Rajput S, Pittman CU, Mohan D (2016) Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J Colloid Interface Sci 468:334–346. doi:10.1016/j.jcis.2015.12.008
Mansourpanah Y, Jafari Z (2015) Efficacy of different generations and concentrations of PAMAM-NH2 on the performance and structure of TFC membranes. React Funct Polym 93:178–189. doi:10.1016/j.reactfunctpolym.2015.04.010
Wu W, Xiao XH, Zhang SF, Zhou JA, Fan LX, Ren F, Jiang CZ (2010) Large-scale and controlled synthesis of iron oxide magnetic short nanotubes: shape evolution, growth mechanism, and magnetic properties. J Phys Chem C 114(39):16092–16103. doi:10.1021/jp1010154
Gandha K, Mohapatra J, Hossain MK, Elkins K, Poudyal N, Rajeshwar K, Liu JP (2016) Mesoporous iron oxide nanowires: synthesis, magnetic and photocatalytic properties. RSC Adv 6(93):90537–90546. doi:10.1039/c6ra18530d
Han XF, Shamaila S, Sharif R, Chen JY, Liu HR, Liu DP (2009) Structural and magnetic properties of various ferromagnetic nanotubes. Adv Mater 21(45):4619–4624. doi:10.1002/adma.200901065
Wu W, Xiao XH, Ren F, Zhang SF, Jiang CZ (2012) A comparative study of the magnetic behavior of single and tubular clustered magnetite nanoparticles. J Low Temp Phys 168(5):306–313. doi:10.1007/s10909-012-0634-3
Dehmlow C, Erhard J, deGroot H (1996) Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology 23(4):749–754. doi:10.1002/hep.510230415
Deep G, Agarwal R (2010) Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastab Rev 29(3):447–463. doi:10.1007/s10555-010-9237-0
Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R (2006) Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer I 98(12):846–855. doi:10.1093/jnci/djj231
Singh RP, Agarwal R (2006) Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog 45(6):436–442. doi:10.1002/mc.20223
Cao X, Deng W, Fu M, Zhu Y, Liu H, Wang L, Zeng J, Wei Y, Xu X, Yu J (2013) Seventy-two-hour release formulation of the poorly soluble drug silybin based on porous silica nanoparticles: in vitro release kinetics and in vitro/in vivo correlations in beagle dogs. Eur J Pharm Sci 48(1–2):64–71. doi:10.1016/j.ejps.2012.10.012
Kumar N, Rai A, Reddy ND, Raj PV, Jain P, Deshpande P, Mathew G, Kutty NG, Udupa N, Rao CM (2014) Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharm Rep 66(5):788–798. doi:10.1016/j.pharep.2014.04.007
Nguyen M-H, Yu H, Dong B, Hadinoto K (2016) A supersaturating delivery system of silibinin exhibiting high payload achieved by amorphous nano-complexation with chitosan. Eur J Pharm Sci 89:163–171. doi:10.1016/j.ejps.2016.04.036
Ripoli M, Angelico R, Sacco P, Ceglie A, Mangia A (2016) Phytoliposome-based silibinin delivery system as a promising strategy to prevent hepatitis C virus infection. J Biomed Nanotechnol 12(4):770–780. doi:10.1166/jbn.2016.2161
Zhou XD, Chen ZP (2015) Preparation and performance evaluation of emulsomes as a drug delivery system for silybin. Arch Pharm Res 38(12):2193–2200. doi:10.1007/s12272-015-0630-7
Xie YC, Yi YN, Hu XW, Shangguan M, Wang LJ, Lu Y, Qi JP, Wu W (2016) Synchronous microencapsulation of multiple components in silymarin into PLGA nanoparticles by an emulsification/solvent evaporation method. Pharm Dev Technol 21(6):672–679. doi:10.3109/10837450.2015.1045616
Cigu TA, Vasiliu S, Racovita S, Lionte C, Sunel V, Popa M, Cheptea C (2016) Adsorption and release studies of new cephalosporin from chitosan-g-poly(glycidyl methacrylate) microparticles. Eur Polym J 82:132–152. doi:10.1016/j.eurpolymj.2016.07.011
Jia H, Kerr LL (2015) Kinetics of drug release from drug carrier of polymer/TiO2 nanotubes composite—pH dependent study. J Appl Polym Sci. doi:10.1002/app.41570
Bravo SA, Lamas MC, Salomón CJ (2002) In-vitro studies of diclofenac sodium controlled-release from biopolymeric hydrophilic matrices. J Pharm Pharm Sci 5(3):213–219
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Acta Pol Pharm 67(3):217–223
Xu S, Yin BR, Guo J, Wang CC (2013) Biocompatible hollow magnetic supraparticles: ultrafast microwave-assisted synthesis, casein-micelle-mediated cavity formation and controlled drug delivery. J Mater Chem B 1(33):4079–4087. doi:10.1039/c3tb20238k
Huang SJ, Ke JH, Chen GJ, Wang LF (2013) One-pot synthesis of PDMAEMA-bound iron oxide nanoparticles for magnetofection. J Mater Chem B 1(43):5916–5924. doi:10.1039/c3tb21149e
Efthimiadou EK, Tapeinos C, Chatzipavlidis A, Boukos N, Fragogeorgi E, Palamaris L, Loudos G, Kordas G (2014) Dynamic in vivo imaging of dual-triggered microspheres for sustained release applications: synthesis, characterization and cytotoxicity study. Int J Pharm 461(1–2):54–63. doi:10.1016/j.ijpharm.2013.11.03
Acknowledgements
Authors thank FONDECYT under Grant No. 1130531. CD thanks CONICYT for her doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chávez, G., Campos, C.H., Jiménez, V.A. et al. Polyamido amine (PAMAM)-grafted magnetic nanotubes as emerging platforms for the delivery and sustained release of silibinin. J Mater Sci 52, 9269–9281 (2017). https://doi.org/10.1007/s10853-017-1140-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1140-4