Abstract
A series of novel magnetic branched polyethylenimine nanogels (Fe3O4/bPEI) was prepared via the combination of crosslinking branched polyethylenimine to form a nanogel and in-situ generation of Fe3O4 on its surface. Morphologies and magnetic properties of the Fe3O4/bPEI nanocomposites could be easily controlled by regulating the ratio between branched polyethylenimine nanogel and Fe3O4 precursor. The nanocomposites could be applied as efficient and selective dye adsorbents for the removal of Congo red under different pH values or in the presence of methylene blue. Furthermore, it could also be applied as catalyst support for the loading of palladium to afford a novel Pd-Fe3O4/bPEI nanocomposite, which exhibited good catalytic activity in the reduction of nitrophenols using NaBH4 as the reducing agent. Particularly, this nanocomposite could be easily separated by an external magnetic field and recycled ten times without appreciable loss of its initial catalytic activity. The synergistic integration of nanogel and magnetic Fe3O4 nanoparticles makes Fe3O4/bPEI to be a versatile platform for multiple applications.
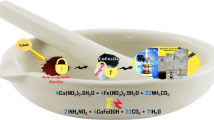
Graphical Abstract
Magnetically recyclable polyethylenimine nanogels (Fe3O4/bPEI), prepared with in-situ generated Fe3O4, can serve as excellent dye adsorbent and catalyst support.











Similar content being viewed by others
References
Ryu JH, Jiwpanich S, Chacko R et al (2010) Surface-functionalizable polymer nanogels with facile hydrophobic guest encapsulation capabilities. J Am Chem Soc 132:8246–8247
Yuan Z, Wang Y, Chen D (2014) Preparation and characterization of thermo-, pH-, and magnetic-field-responsive organic/inorganic hybrid microgels based on poly (ethylene glycol). J Mater Sci 49:3287–3296. doi:10.1007/s10853-014-8037-2
Zhang X, Malhotra S, Molina M et al (2015) Micro-and nanogels with labile crosslinks–from synthesis to biomedical applications. Chem Soc Rev 44:1948–1973
Xiong Y, Wang H, Wang R et al (2010) A facile one-step synthesis to cross-linked polymeric nanoparticles as highly active and selective catalysts for cycloaddition of CO2 to epoxides. Chem Commun 46:3399–3401
Moore BL, Moatsou D, Lu A et al (2014) Studying the activity of the MacMillan catalyst embedded within hydrophobic cross-linked polymeric nanostructures. Polym Chem 10:3487–3494
Li B, Jiang X, Yin J (2012) Multi-responsive microgel of hyperbranched poly(ether amine)(hPEA-mGel) for the selective adsorption and separation of hydrophilic fluorescein dyes. J Mater Chem 22:17976–17983
Bhardwaj P, Singh V, Mandal UK et al (2010) Polyacrylamide and poly (acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid)-silica composite nanogels through in situ microemulsion polymerisation. J Mater Sci 45:1008–1016. doi:10.1007/s10853-009-4032-4
Kaewsaneha C, Tangboriboonrat P, Polpanich D et al (2013) A. Janus colloidal particles: preparation, properties, and biomedical applications. ACS Appl Mater Interfaces 5:1857–1869
Sasaki Y, Akiyoshi K (2010) Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem Rec 10:366–376
Wu W, Mitra N, Yan EC et al (2010) Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano 4:4831–4839
Smith MH, Lyon LA (2011) Multifunctional nanogels for siRNA delivery. Acc Chem Res 45:985–993
Argentiere S, Blasi L, Morello G et al (2011) A novel pH-responsive nanogel for the controlled uptake and release of hydrophobic and cationic solutes. J Phys Chem C 115:16347–16353
Lu A, Moatsou D, Longbottom DA et al (2013) Tuning the catalytic activity of l-proline functionalized hydrophobic nanogel particles in water. Chem Sci 4:965–969
Bonomi P, Servant A, Resmini M (2012) Modulation of imprinting efficiency in nanogels with catalytic activity in the Kemp elimination. J Mol Recognit 25:352–360
Oishi M, Miyagawa N, Sakura T et al (2007) pH-responsive PEGylated nanogel containing platinum nanoparticles: application to on–off regulation of catalytic activity for reactive oxygen species. React Funct Polym 67:662–668
Pourjavadi A, Nazari-Chamazkoti M, Hosseini SH (2015) Polymeric ionic liquid nanogel-anchored tungstate anions: a robust catalytic system for oxidation of sulfides to sulfoxides. New J Chem 39:1348–1354
Sui ZY, Cui Y, Zhu JH et al (2013) Preparation of three-dimensional graphene oxide–polyethylenimine porous materials as dye and gas adsorbents. ACS Appl Mater Interfaces 5:9172–9179
Ma Y, Liu WJ, Zhang N et al (2014) Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresource Technol 169:403–408
Heydari-Gorji A, Sayari A (2012) Thermal, oxidative, and CO2-induced degradation of supported polyethylenimine adsorbents. Ind Eng Chem Res 51:6887–6894
Wang ML, Jiang TT, Lu Y et al (2013) Gold nanoparticles immobilized in hyperbranched polyethylenimine modified polyacrylonitrile fiber as highly efficient and recyclable heterogeneous catalysts for the reduction of 4-nitrophenol. J Mater Chem A 1:5923–5933
Zhang X, Zhang X, Yang B et al (2014) Biocompatible fluorescent organic nanoparticles derived from glucose and polyethylenimine. Colloid Surface B 123:747–752
Lupitskyy R, Minko S (2010) Robust synthesis of nanogel particles by an aggregation-crosslinking method. Soft Matter 6:4396–4402
Sun X, Yang L, Xing H et al (2013) Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chem Eng J 234:338–345
Fan Y, Liu HJ, Zhang Y et al (2015) Adsorption of anionic MO or cationic MB from MO/MB mixture using polyacrylonitrile fiber hydrothermally treated with hyperbranched polyethylenimine. J Hazard Mater 283:321–328
Lei Y, Chen F, Luo Y et al (2014) Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr(VI) removal. J Mater Sci 49:4236–4245. doi:10.1007/s10853-014-8118-2
Xie Y, Yan B, Xu H et al (2014) Highly regenerable mussel-inspired Fe3O4@polydopamine-Ag core–shell microspheres as catalyst and adsorbent for methylene blue removal. ACS Appl Mater Interfaces 6:8845–8852
Mu B, Wang A (2015) One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support. J Mater Chem A 3:281–289
Mohammadi A, Barikani M, Barmar M (2013) Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J Mater Sci 48:7493–7502. doi:10.1007/s10853-013-7563-7
Xie W, Wang J (2014) Enzymatic production of biodiesel from soybean oil by using immobilized lipase on Fe3O4/poly(styrene-methacrylic acid) magnetic microsphere as a biocatalyst. Energy Fuel 28:2624–2631
Cai Y, Jiang JS, Zheng B et al (2013) Synthesis and properties of magnetic sensitive shape memory Fe3O4/poly(ε-caprolactone)-polyurethane nanocomposites. J Appl Polym Sci 127:49–56
Cui Q, Zhu S, Yan Y et al (2015) Superparamagnetic Fe3O4/poly(N-isopropyl acrylamide) nanocomposites synthesized in inverse miniemulsions: magnetic and particle properties. J Nanosci Nanotechnol 15:4608–4618
Zhao L, Liu H, Wang F et al (2014) Design of yolk–shell Fe3O4@PMAA composite microspheres for adsorption of metal ions and pH-controlled drug delivery. J Mater Chem A 2:7065–7074
Liu B, Zhang W, Yang F et al (2011) Facile method for synthesis of Fe3O4@polymer microspheres and their application as magnetic support for loading metal nanoparticles. J Phys Chem C 115:15875–15884
Guo W, Wang Q, Wang G et al (2013) Facile hydrogen-bond-assisted polymerization and immobilization method to synthesize hierarchical Fe3O4@poly(4-vinylpyridine-co-divinylbenzene)@Au nanostructures and their catalytic applications. Chem Asian J 8:1160–1167
Xuan S, Wang YXJ, Leung KCF et al (2008) Synthesis of Fe3O4@polyaniline core/shell microspheres with well-defined blackberry-like morphology. J Phys Chem C 112:18804–18809
Liu R, Guo Y, Odusote G et al (2013) Core–shell Fe3O4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent. ACS Appl Mater Interfaces 5:9167–9171
Tian Q, Wang Q, Yao KX et al (2014) Multifunctional polypyrrole@Fe3O4 nanoparticles for dual-modal imaging and in vivo photothermal cancer therapy. Small 10:1063–1068
Zhao M, Deng K, He L et al (2014) Core–shell palladium nanoparticle@metal–organic frameworks as multifunctional catalysts for cascade reactions. J Am Chem Soc 136:1738–1741
Xue Y, Chen H, Yu D et al (2011) Oxidizing metal ions with graphene oxide: the in situ formation of magnetic nanoparticles on self-reduced graphene sheets for multifunctional applications. Chem Commun 47:11689–11691
Yoon T, Chae C, Sun YK et al (2011) Bottom-up in situ formation of Fe3O4 nanocrystals in a porous carbon foam for lithium-ion battery anodes. J Mater Chem 21:17325–17330
Karami B, Hoseini SJ, Eskandari K et al (2012) Synthesis of xanthene derivatives by employing Fe3O4 nanoparticles as an effective and magnetically recoverable catalyst in water. Catal Sci Technol 2:331–338
Beyth N, Yudovin-Farber I, Bahir R et al (2006) Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 27:3995–4002
Santoyo Salazar J, Perez L, de Abril O et al (2011) Magnetic iron oxide nanoparticles in 10–40 nm range: composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem Mater 23:1379–1386
Lee H, Lee E, Kim DK et al (2006) Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc 128:7383–7389
Liang J, Ma H, Luo W et al (2013) Synthesis of magnetite submicrospheres with tunable size and superparamagnetism by a facile polyol process. Mater Chem Phys 139:383–388
Zhang X, Jiang L (2011) Fabrication of novel rattle-type magnetic mesoporous carbon microspheres for removal of microcystins. J Mater Chem 21:10653–10657
Patil K, Pawar R, Talap P (2000) Self-aggregation of methylene blue in aqueous medium and aqueous solutions of Bu4NBr and urea. Phys Chem Chem Phys 2:4313–4317
Ribeiro SM, Serra AC, Gonsalves ADAR (2011) Silica grafted polyethylenimine as heterogeneous catalyst for condensation reactions. Appl Catal A 399:126–133
Long W, Brunelli NA, Didas SA et al (2013) Aminopolymer–silica composite-supported Pd catalysts for selective hydrogenation of alkynes. ACS Catal 3:1700–1708
Yang J, Tian C, Wang L (2011) An effective strategy for small-sized and highly-dispersed palladium nanoparticles supported on graphene with excellent performance for formic acid oxidation. J Mater Chem 21:3384–3390
Wen X, Li G, Chen Q et al (2014) Organic-soluble palladium nanoparticles costabilized by hyperbranched polymer and dispersants as highly efficient and reusable catalysts in biphasic solution. Ind Eng Chem Res 53:11646–11652
Rashid M, Mandal TK (2008) Templateless synthesis of polygonal gold nanoparticles: an unsupported and reusable catalyst with superior activity. Adv Funct Mater 18:2261–2271
Li H, Gao S, Zheng Z et al (2011) Bifunctional composite prepared using layer-by-layer assembly of polyelectrolyte-gold nanoparticle films on Fe3O4-silica core-shell microspheres. Catal Sci Technol 1:1194–1201
Esumi K, Isono R, Yoshimura T (2004) Preparation of PAMAM − and PPI − metal (silver, platinum, and palladium) nanocomposites and their catalytic activities for reduction of 4-nitrophenol. Langmuir 20:237–243
Mei Y, Lu Y, Polzer F et al (2007) Catalytic activity of palladium nanoparticles encapsulated in spherical polyelectrolyte brushes and core-shell microgels. Chem Mater 19:1062–1069
Chang Y, Chen D (2009) Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J Hazard Mater 165:664–669
Kuroda K, Ishida T, Haruta M (2009) Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J Mol Catal A 298:7–11
Lu X, Bian X, Nie G et al (2012) Encapsulating conducting polypyrrole into electrospun TiO2 nanofibers: a new kind of nanoreactor for in situ loading Pd nanocatalysts towards p-nitrophenol hydrogenation. J Mater Chem 22:12723–12730
Liu Y, Fan Y, Yuan Y et al (2012) Amphiphilic hyperbranched copolymers bearing a hyperbranched core and a dendritic shell as novel stabilizers rendering gold nanoparticles with an unprecedentedly long lifetime in the catalytic reduction of 4-nitrophenol. J Mater Chem 22:21173–21182
El-Sheikh SM, Ismail AA, Al-Sharab JF (2013) Catalytic reduction of p-nitrophenol over precious metals/highly ordered mesoporous silica. New J Chem 37:2399–2407
Acknowledgements
The authors thank Professor Gang Ma for his kind help. Financial support by the National Natural Science Foundation of China (21376060), the Natural Science Foundation of Hebei Province (B2014201024), and the Youth Foundation of Hebei Educational Committee (QN2014069) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2015_9627_MOESM1_ESM.docx
TEM image and photograph of magnetic separation of the comparative sample of Fe3O4(2)/bPEI; TEM image and photograph of magnetic separation of Fe3O4(2)-bPEI; FTIR analysis of bPEI-HCl nanogel; TEM image and the size distribution of pure Fe3O4 (inset); UV–vis spectra of the mixture solution of dyes before and after adsorption; TEM, STEM, and HAADF-STEM images of Pd-Fe3O4(2)/bPEI with elemental mappings; XRD pattern of Pd-Fe3O4(2)/bPEI; photograph of the solution of 4-NP before and after reduction. Supplementary material 1 (DOCX 8831 kb)
Rights and permissions
About this article
Cite this article
Wen, X., Qiao, X., Han, X. et al. Multifunctional magnetic branched polyethylenimine nanogels with in-situ generated Fe3O4 and their applications as dye adsorbent and catalyst support. J Mater Sci 51, 3170–3181 (2016). https://doi.org/10.1007/s10853-015-9627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9627-3