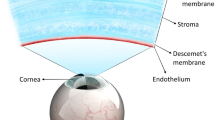
Abstract
Synthetic materials have played a significant role in ophthalmic applications to improve vision for many years. This has been in four main areas in ophthalmology: ocular surface reconstruction, lens replacement, vitreous replacement and structural support and cell transplantation in the retina. Corneal replacement therapies have been developed using both synthetic acrylic-based materials and more recently naturally derived materials such as amniotic membrane. Intraocular lenses as a replacement for the natural lens post cataract surgery has been used for many years. Newer developments include the opportunity to use gels so that the lenses can accommodate but these need improving in terms of the cross-linking chemistry. Silicone oils have been used as long-term tamponade agents as vitreous replacements but recent developments in their properties has enhanced the clinical outcomes and further research into their use as drug delivery vehicles will be a major advancement. Regenerative medicine therapies to repopulate the retina to repair and replace specific cell layers require the optimisation of synthetic scaffolds and this is a major area for development. Recent developments in biomaterials have emphasised the importance of the physical, chemical and mechanical properties specific to a particular ophthalmic application. Materials science has a critical role in developing strategies to overcome vision loss.



Similar content being viewed by others
References
Williams RL, Wong D (2009) Ophthalmic biomaterials. In: Narayan R (ed) Biomedical Materials. Springer, New York, pp 327–348
Kearns V et al (2012) Ophthalmic applications of biomaterials in regenerative medicine. In: Ramalingam M, Ramakrishna S, Best S (eds) Biomaterials and stem cells in regnerative medicine. CRC Press, Boca Raton, pp 185–218
Mason SL et al (2011) Ocular epithelial transplantation: current uses and future potential. Regen Med 6(6):767–782
Schermer A, Galvin S, Sun TT (1986) Differentiation-related expression of a major 64 K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103(1):49–62
Bray LJ et al (2014) Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy 16(1):64–73
Walshe J, Harkin DG (2014) Serial explant culture provides novel insights into the potential location and phenotype of corneal endothelial progenitor cells. Exp Eye Res 127:9–13
Hicks CR et al (1997) Keratoprostheses: advancing toward a true artificial cornea. Surv Ophthalmol 42(2):175–189
Nguyen P, Yiu SC (2008) Ocular surface reconstruction: recent innovations, surgical candidate selection and postoperative management. Expert Rev Ophthalmol 3(5):567–584
Rimmer S et al (2007) Epithelialization of hydrogels achieved by amine functionalization and co-culture with stromal cells. Biomaterials 28(35):5319–5331
Evans MDM, Sweeney DF (2010) Synthetic corneal implants. In: Chirila TV (ed) Biomaterials and regenerative medicine in ophthalmology. Woodhead Publishing Limited, Cambridge, pp 65–133
Yiu SC, Thomas PB, Nguyen P (2007) Ocular surface reconstruction: recent advances and future outlook. Curr Opin Ophthalmol 18(6):509–514
Koizumi N et al (2000) Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci 41(9):2506–2513
Tseng, S.C., et al (2002) Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. Adv Ex Med Biol 506(Pt B): 1323–1334
Nishida K et al (2004) Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 77(3):379–385
Yokoo S et al (2008) Human corneal epithelial equivalents for ocular surface reconstruction in a complete serum-free culture system without unknown factors. Invest Ophthalmol Vis Sci 49(6):2438–2443
Pellegrini G et al (1997) Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349(9057):990–993
Schwab IR (1999) Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc 97:891–986
Baylis O et al (2011) 13 Years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem 112(4):993–1002
Rama P et al (2010) Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363(2):147–155
Han B et al (2002) A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea 21(5):505–510
Talbot M et al (2006) Autologous transplantation of rabbit limbal epithelia cultured on fibrin gels for ocular surface reconstruction. Mol Vis 12:65–75
Dravida S et al (2008) A biomimetic scaffold for culturing limbal stem cells: a promising alternative for clinical transplantation. J Tissue Eng Regen Med 2(5):263–271
Levis HJ, Brown RA, Daniels JT (2010) Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials 31(30):7726–7737
Mi S et al (2010) Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J Biomed Mater Res Part A 95(2):447–453
Levis HJ et al (2013) Plastic compressed collagen constructs for ocular cell culture and transplantation: a new and improved technique of confined fluid loss. Curr Eye Res 38(1):41–52
Rafat M et al (2008) PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 29(29):3960–3972
Reichl S, Borrelli M, Geerling G (2011) Keratin films for ocular surface reconstruction. Biomaterials 32(13):3375–3386
Bray LJ et al (2011) Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials 32(22):5086–5091
Chirila TV et al (2008) Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng Part A 14(7):1203–1211
Higa K et al (2011) Porous silk fibroin film as a transparent carrier for cultivated corneal epithelial sheets. J Biomater Sci Polym Ed 22(17):2261–2276
Deshpande P et al (2009) Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng Part A 15(10):2889–2902
Deshpande P et al (2013) Simplifying corneal surface regeneration using a biodegradable synthetic membrane and limbal tissue explants. Biomaterials 34(21):5088–5106
Ortega Í et al (2013) Development of a microfabricated artificial limbus with micropockets for cell delivery to the cornea. Biofabrication 5(2)
Ortega Í et al (2013) Combined microfabrication and electrospinning to produce 3-D architectures for corneal repair. Acta Biomater 9(3):5511–5520
Deshpande P et al (2013) Cultivation of limbal epithelial cells on electrospun poly (lactide-co-glycolide) scaffolds for delivery to the cornea. In: Wright B, Connon CJ (eds) Corneal Regenerative Medicine. Humana Press, New Jersey, pp 179–185
Ishino Y et al (2004) Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci 45(3):800–806
Ide T et al (2006) Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials 27(4):607–614
Nitschke M et al (2007) Thermo-responsive poly(NiPAAm-co-DEGMA) substrates for gentle harvest of human corneal endothelial cell sheets. J Biomed Mater Res, Part A 80A(4):1003–1010
Lai J-Y, Chen K-H, Hsiue G-H (2007) Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 84(10):1222–1232
Levis HJ et al (2012) Plastic compressed collagen as a novel carrier for expanded human corneal endothelial cells for transplantation. PLoS ONE 7(11)
Crabb RA et al (2006) Biomechanical and microstructural characteristics of a collagen film-based corneal stroma equivalent. Tissue Eng 12(6):1565–1575
Tonsomboon K, Oyen ML (2013) Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J Mech Behav Biomed Mater 21:185–194
Lai JY (2013) Corneal stromal cell growth on gelatin/chondroitin sulfate scaffolds modified at different nhs/edc molar ratios. Int J Mol Sci 14(1):2036–2055
Hu X et al (2005) Tissue engineering of nearly transparent corneal stroma. Tissue Eng 11(11–12):1710–1717
Salehi S et al (2014) Generation of PGS/PCL blend nanofibrous scaffolds mimicking corneal stroma structure. Macromol Mater Eng 299(4):455–469
Wu J et al (2012) The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 33(5):1343–1352
Schrader S et al (2009) Tissue engineering for conjunctival reconstruction: established methods and future outlooks. Curr Eye Res 34(11):913–924
Ang LPK, Tan DTH (2005) Autologous cultivated conjunctival transplantation for recurrent viral papillomata. Am J Ophthalmol 140(1):136–138
Resnikoff S et al (2004) Policy and practice, global data on visual impairment in the year 2002. Bull World Health Organ 82(11):844–851
Taylor H (2000) Cataract: how much surgery do we have to do? Br J Ophthalmol 84(1):1–2
Patel AS (2004) Introduction to optics of the eye, cataracts, and intraocular lens implants. In: Ratner BD et al (eds) Biomaterials science: an introduction to materials in medicine. Elsevier Academic Press, London, pp 591–602
Refojo MF (2004) Ophthalmic applications. In: Ratner BD et al (eds) Biomaterials science: an introduction to materials in medicine. Elsevier Academic Press, London, pp 583–591
Hollick EJ et al (1998) Lens epithelial cell regression on the posterior capsule with different intraocular lens materials. Br J Ophthalmol 82(10):1182–1188
Williams RL, Kearns VR (2010) Drug-device combination products for ocular applications. In: Lewis A (ed) Drug-device combination products: delivery technologies and applications. Woodhead Publishing Limited, Cambridge, pp 311–340
Saika S (2004) Relationship between posterior capsule opacification and intraocular lens biocompatibility. Prog Retin Eye Res 23(3):283–305
Ohadi C, Moreira H, McDonnell PJ (1991) Posterior capsule opacification. Curr Opin Ophthalmol 2(1):46–52
Yuen C et al (2006) Modification of the surface properties of a lens material to influence posterior capsule opacification. Clin Exp Ophthalmol 34(1):568–574
Schaumberg DA et al (1998) A systematic overview of the incidence of posterior capsule opacification. Opthalmology 105(7):1213–1221
Nishi O, Nishi K, Sakanishi K (1998) Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens. Ophthalmic Surg Lasers Imaging 29:587–594
Oshika T, Nagata T, Ishii Y (1998) Adhesion of lens capsule to intraocular lenses of polymethylmethacrylate, silicone, and acrylic foldable materials: an experimental study. Br J Ophthalmol 82:549–553
Nishi O, Nishi K (1999) Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. J Cataract Refract Surg 25:1–6
Peng Q et al (2000) Surgical prevention of posterior capsule opacification: part 3: Intraocular lens optic barrier effect as a second line of defense. J Cataract Refract Surg 26(2):198–213
Ayaki M et al (2003) Lens epithelial cell migration between posterior capsule and intraocular lens with variously finished posterior optic edge and two haptic angulations. Ophthalmic Res 35(5):261–267
Nishi O, Nishi K, Wickstrom K (2000) Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg 26:1543–1549
Gayton JL et al (2000) Interlenticular opacification: clinicopathological correlation of a complication of posterior chamber piggyback intraocular lenses. J Cataract Refract Surg 26(3):330–336
Linnola RJ et al (2000) Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes: part 1: histological sections. J Cataract Refract Surg 26(12):1792–1806
Linnola RJ et al (2000) Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes: Part 2: explanted intraocular lenses. J Cataract Refract Surg 26(12):1807–1818
Linnola RJ (1997) Sandwich theory: Bioactivity-based explanation for posterior capsule opacification. J Cataract Refract Surg 23(10):1539–1542
Linnola RJ et al (2003) Adhesion of soluble fibronectin, vitronectin, and collagen type IV to intraocular lens materials. J Cataract Refract Surg 29(1):146–152
D’Sa RA et al (2011) Inhibition of lens epithelial cell growth via immobilisation of hyaluronic acid on atmospheric pressure plasma modified polystyrene. Soft Matter 7(2):608–617
D’Sa RA, Burke GA, Meenan BJ (2010) Lens epithelial cell response to atmospheric pressure plasma modified poly(methylmethacrylate) surfaces. J Mater Sci 21(5):1703–1712
Yao K et al (2006) Improvement of the surface biocompatibility of silicone intraocular lens by the plasma-induced tethering of phospholipid moieties. J Biomed Mater Res Part A 78(4):684–692
Lundvall A et al (2003) Effect of 3-piece AcrySof and downsized heparin-surface-modified poly(methyl methacrylate) intraocular lenses in infant rabbit eyes. J Cataract Refract Surg 29(1):159–163
Arima Y, Iwata H (2007) Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 28(20):3074–3082
D’Sa RA, Burke GA, Meenan BJ (2010) Protein adhesion and cell response on atmospheric pressure dielectric barrier discharge-modified polymer surfaces. Acta Biomater 6(7):2609–2620
Khatua D et al (2011) Influence of charge densities of randomly sulfonated polystyrene surfaces on cell attachment and proliferation. J Nanosci Nanotechnol 11(5):4227–4230
Ratner BD (2004) Correlation, surfaces and biomaterials science. In: Ratner BD et al (eds) Biomaterials science: an introduction to materials in medicine. Elsevier Academic Press, London, pp 765–771
France RM et al (1998) Attachment of human keratinocytes to plasma co-polymers of acrylic acid/octa-1,7-diene and allyl amine/octa-1,7-diene. J Mater Chem 8(1):37–42
Jacchetti E et al (2008) Biomimetic poly(amidoamine) hydrogels as synthetic materials for cell culture. J Nanobiotechnol 6(14):14–28
Kearns V et al (2012) Plasma polymer coatings to aid retinal pigment epithelial growth for transplantation in the treatment of age related macular degeneration. J Mater Sci 23(8):2013–2021
Uygun BE, Stojsih SE, Matthew HWT (2009) Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng Part A 15(11):3499–3512
Orlidge A, D’Amore PA (1986) Cell specific effects of glycosaminoglycans on the attachment and proliferation of vascular wall components. Microvasc Res 31(1):41–53
Hao X et al (2010) Functionalised polysiloxanes as injectable, in situ curable accommodating intraocular lenses. Biomaterials 31(32):8153–8163
Hao X et al (2012) High refractive index polysiloxane as injectable, in situ curable accommodating intraocular lens. Biomaterials 33(23):5659–5671
Annaka M et al (2012) Organic-inorganic nanocomposite gels as an in situ gelation biomaterial for injectable accommodative intraocular lens. Soft Matter 8(27):7185–7196
Baino F (2011) Towards an ideal biomaterial for vitreous replacement: historical overview and future trends. Acta Biomater 7(3):921–935
Fawcett IM, Williams RL, Wong D (1994) Contact angles of substances used for internal tamponade in retinal detachment surgery. Graefe’s Arch Clin Exp Ophthalmol 232(7):438–444
Williams R, Wong D (1999) The influence of explants on the physical efficiency of tamponade agents. Graefes Arch Clin Exp Ophthalmol 237(10):870–874
Scott IU et al (2005) Outcomes of complex retinal detachment repair using 1000- vs 5000-centistoke silicone oil. Arch Ophthalmol 123(4):473–478
Ichhpujani P, Jindal A, Jay Katz L (2009) Silicone oil induced glaucoma: a review. Graefes Arch Clin Exp Ophthalmol 247(12):1585–1593
Williams RL, Stappler T (2003) Emulsification of tamponade agents. In: LoisN, Wong D (Eds) Complications of vitreo-retinal surgery, Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia. p. 224–231
Caramoy A et al (2011) Development of emulsification-resistant silicone oils: can we go beyond 2000 mPas silicone oil? Invest Ophthalmol Vis Sci 52(8):5432–5436
Caramoy A et al (2010) In vitro emulsification assessment of new silicone oils. Br J Ophthalmol 94(4):509–512
Williams RL et al (2010) Increasing the extensional viscosity of silicone oil reduces the tendency for emulsification. Retina 30(2):300–304
Williams RL et al (2011) Injectability of silicone oil-based tamponade agents. Br J Ophthalmol 95(2):273–276
Kleinberg TT et al (2011) Vitreous substitutes: a comprehensive review. Surv Ophthalmol 56(4):300–323
Wetterqvist C et al (2004) Tamponade efficiency of perfluorohexyloctane and silicone oil solutions in a model eye chamber. Br J Ophthalmol 88(5):692–696
Lai WW et al (2008) Emulsification and inverted hypopyon formation of oxane HD in the anterior chamber. Graefe’s Arch Clin Exp Ophthalmol 246(11):1633–1635
Li W et al (2010) Clinical results of densiron 68 intraocular tamponade for complicated retinal detachment. Ophthalmologica 224(6):354–360
Wang XD (2011) Analysis of clinical complications of Densiron68 as an intraocular tamponade in the vitreoretinal surgery. Int J Ophthalmol 11(7):1227–1229
Wickham L et al (2010) The use of silicone oil-RMN3 (Oxane HD) as heavier-than-water internal tamponade in complicated inferior retinal detachment surgery. Graefe’s Arch Clin Exp Ophthalmol 248(9):1225–1231
Williams RL et al (2013) Novel heavy tamponade for vitreoretinal surgery. Invest Ophthalmol Vis Sci 54(12):7284–7292
Le Goff MM, Bishop PN (2008) Adult vitreous structure and postnatal changes. Eye 22(10):1214–1222
Zimmerman RL (1980) In vivo measurements of the viscoelasticity of the human vitreous humor. Biophys J 29(3):539–544
Hong Y et al (1998) Biodegradation in vitro and retention in the rabbit eye of crosslinked poly(1-vinyl-2-pyrrolidinone) hydrogel as a vitreous substitute. J Biomed Mater Res 39(4):650–659
Daniele S et al (1968) Glyceryl methacrylate hydrogel as a vitreous implant: an experimental study. Arch Ophthalmol 80(1):120–127
Refojo MF (1971) Polymers in ophthalmic surgery. J Biomed Mater Res 5(1):113–119
Hogen-Esch TE, Shah KR, Fitzgerald CR (1976) Development of injectable poly(glyceryl methacrylate) hydrogels for vitreous prosthesis. J Biomed Mater Res 10(6):975–976
Swindle KE, Hamilton PD, Ravi N (2006) Advancements in the development of artifical vitreous humor utilizing polyacrylamide copolymers with disulfide crosslinkers. Polym Prepr 47(1):56–60
Foster WJ et al (2006) Internal osmotic pressure as a mechanism of retinal attachment in a vitreous substitute. J Bioact Compat Polym 21(3):221–235
Swindle KE, Hamilton PD, Ravi N (2008) In situ formation of hydrogels as vitreous substitutes: viscoelastic comparison to porcine vitreous. J Biomed Mater Res, Part A 87A(3):656–665
Yamauchi A (1991) Synthetic vitreous body of PVA hydrogel. In: DeRossi D et al (ed) Polymer gels, Springer, New York. p. 127–134
Leone G et al (2010) PVA/STMP based hydrogels as potential substitutes of human vitreous. J Mater Sci 21(8):2491–2500
Reichenbach A, Bringmann A (2013) New functions of Müller cells. Glia 61(5):651–678
Morgan JE (2004) Circulation and axonal transport in the optic nerve. Eye 18(11): p. 1089–1095
Kador Karl E et al (2013) Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials 34:4242–4250
Lavik EB et al (2005) Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials 26(16):3187–3196
McUsic AC, Lamba DA, Reh TA (2012) Guiding the morphogenesis of dissociated newborn mouse retinal cells and hES cell-derived retinal cells by soft lithography-patterned microchannel PLGA scaffolds. Biomaterials 33(5):1396–1405
Redenti S et al (2008) Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(ε-caprolactone) nanowire scaffold. J Ocul Biol Dis Infor 1(1):19–29
Tao S et al (2007) Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip 7(6):695–701
Thumann G et al (2009) The in vitro and in vivo behaviour of retinal pigment epithelial cells cultured on ultrathin collagen membranes. Biomaterials 30(3):287–294
Lu JT et al (2007) Thin collagen film scaffolds for retinal epithelial cell culture. Biomaterials 28(8):1486–1494
Nicolini J et al (2000) The anterior lens capsule used as support material in RPE cell-transplantation. Acta Ophthalmol Scand 78(5):527–531
Capeáns C et al (2003) Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand 81(3):271–277
Stanzel BV et al (2005) Amniotic membrane maintains the phenotype of rabbit retinal pigment epithelial cells in culture. Exp Eye Res 80(1):103–112
Ohno-Matsui Kyoko et al (2006) In vitro and in vivo characterization of iris pigment epithelial cells cultured on amniotic membranes. Mol Vis 12:1022–1032
Davis AA et al (1995) A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci 36(5):955–964
Warnke PH et al (2013) Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater 9(12):9414–9422
Cai S et al (2012) Mouse retinal progenitor cell dynamics on electrospun poly (ε-caprolactone). J Biomater Sci Polym Ed 23(11):1451–1465
Tomita M et al (2005) Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells 23(10):1579–1588
Christiansen AT et al (2012) Subretinal implantation of electrospun, short nanowire, and smooth poly(ε-caprolactone) scaffolds to the subretinal space of porcine eyes. Stem Cells Int
Liu Z et al (2014) Enhancement of retinal pigment epithelial culture characteristics and subretinal space tolerance of scaffolds with 200 nm fiber topography. Biomaterials 35(9):2837–2850
Williams RL et al (2005) Polyurethanes as potential substrates for sub-retinal retinal pigment epithelial cell transplantation. J Mater Sci 16(12):1087–1092
Krishna Y et al (2007) Polydimethylsiloxane as a substrate for retinal pigment epithelial cell growth. J Biomed Mater Res, Part A 80A(3):669–678
Krishna Y et al (2011) Expanded polytetrafluoroethylene as a substrate for retinal pigment epithelial cell growth and transplantation in age-related macular degeneration. Br J Ophthalmol 95(4):569–573
Kearns V et al (2012) Plasma polymer coatings to aid retinal pigment epithelial growth for transplantation in the treatment of age related macular degeneration. J Mater Sci 23(8):2013–2021
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lace, R., Murray-Dunning, C. & Williams, R. Biomaterials for ocular reconstruction. J Mater Sci 50, 1523–1534 (2015). https://doi.org/10.1007/s10853-014-8707-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8707-0