Abstract
The low stability and complicated fabrication procedures seriously hindered practical applications of superhydrophobic and superoleophilic materials. Here, we present a simple method for preparing the novelly three-dimensional material based on commercially available nickel foams functionalized with electrodepositing of sub-micrometer polypyrrole (PPy) particles, followed by modification of low-surface-energy material such as fluoroalkylsilane (FAS), which can efficiently separate oils and organic solvents from water. The formation of nanostructured surface roughness of PPy onto the nickel foam by combination with FAS modification would contribute to the excellent superhydrophobic and superoleophilic performance, as is the evidence of the water CA of 155° and oil CA of ca. 0° for FAS-treated PPy foam. As a separating membrane, organic solvents and oils could be easily removed without obvious absorption of water, which has great potential over traditional treatment techniques and is of technological significance as a promising and efficient absorbent material for separation of organic contaminates and oils from water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pollution of groundwater and seawater has become a severely environmental hazard as the result of oil spillage and industrial waste such as chemicals and pesticides [1]. To address environmental issues, such development of novel strategies with high separation capacity, high selectivity, and stable performance for removal of oil spillage or industrial contaminants should be of significant importance and urgently needed. So far, many conventional methods such as gravity separation, ultrasonic separation, and absorption technology [2–6] have been widely used in application of oils (organics)/water separation. Among absorbent materials, many kinds of superwetting materials such as sponges [7–10], superhydrophobic graphene [11–20], carbons [21–23], superwetting inorganic (organic) nanowire membranes [24–30], microporous polymers [31], and macroporous gels [32–34] et al. have been reported to have excellent absorption selectivity for removal of organics and oils from water [8, 35–37]. The first case of oils/water separation has been reported by Jiang and coworkers [2] using a mesh with coating of superoleophilic materials. Along this line, the creation of nanometer- or micrometer-sized porous materials with good superhydrophobic surface has more recently been demonstrated for successful use in the separation and absorption of oils and organic solvents from water. In our previous studies, we also reported the preparation of superhydrophobic polymer [38], microporous absorbents [39, 40], and mesh film [41] for removal of oils and organic solvents from water. Quite different from some traditional absorbents such as active carbon [42] and clay [43, 44], these superhydrophobic absorbents or meshes show excellent absorption selectivity, high absorbency and recyclability for oils (organics)/water separation. In most cases, the needing of complicated fabrication techniques is a major obstacle which hinders their large-scale practical application. Based on this point, the exploring of simple method for preparation of superwetting absorbents for oils (organics)/water separation should be of most importance to address this problem. In this work, the preparation of superhydrophobic and superoleophilic foam with simple coating of conductive polymer by electrodepositing method was reported, followed by modification with low-surface-energy material, such as fluoroalkylsilane (FAS), for organics/oils and water separation. As a membrane for oils (organics)/water separation, the oils, and organics can be continually removed from water with high absorption selectivity, which would have great potential in applications of water treatment and oil spillage cleanup, etc.
Experimental section
Preparation of PPy foam
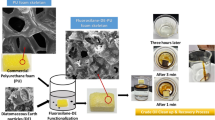
Pyrrole (99 %, J&K) was purified and stored below 0 °C before using. Commercial nickel foam as working electrode was ultrasonic ion treated with aqueous NaOH (1 M), distilled water and ethanol, respectively. A plate of platinum (size: 15 mm × 15 mm × 0.2 mm) was used as counter electrode after being washed by HCl (1 M) and distilled water. The electrodepositing solution contained pyrrole (0.14 M) and SDBS (0.015 M) and 100 mL distilled water. A small amount of NaClO4 (0.1 mg) was used as the oxidant during the electrodepositing process. PPy was obtained on the surface of nickel foam with two-electrode system at 11.5 V for 3 h (Fig. 1a). The resulted PPy foam was washed with distilled water, acetone and air-dried.
Surface modification
The resulted PPy foam was treated by dip-and-dry method, which was repeatedly dipped into a FAS/toluene solution (3 wt%) and dried in an oven at 80 °C for 12 h.
Characterization
The morphology of the PPy foam was measured by scanning electron microscopy (SEM, JSM-7601F). Solid Fourier transform infrared spectra (FT-IR) were recorded in the range of 4000–400 cm−1 using KBr pellet technique on a FT-Raman Module (Nicolet, America) instrument. X-ray photoelectron spectroscopy (XPS) analysis was performed on an ESCALAB 250xi spectrometer (Thermon Scientific). Water contact angle (CA) measurement was performed on a CA meter (DSA100, Kruss).
Results and discussion
In this work, we used commercial nickel foam as an electrode for fabrication of PPy foam. Polypyrrole is a heterocyclic kind of conjugate polymer materials, which has been widely used in field of oil/water separation [45–47]. With the simple electrodepositing method, a new kind of three-dimensional (3D) PPy material based on the nickel foam was fabricated, as shown in Fig. 1a. SEM was performed to evaluate the morphology of PPy material. From Fig. 1b, the as-treated commercial nickel foam shows a macroporous structure with smooth surface morphology (Fig. 1b, inset). After coating with PPy particles, as seen in Fig. 1c, the PPy film on the nickel foam exhibits lotus-like structure morphology with good roughness and highly porosity [48–50]. Higher magnification SEM image (Fig. 1c, inset) shows the PPy foam with thin films in diameter range of 4–5 μm, which is consisted of micrometer-sized PPy particles with average size of 8 μm.
The surface wettability was evaluated by CA measurement. With the hydrophilic reason of PPy in nature, the proposed PPy foam shows a superhydrophilic property with a water CA of ca. 0°. Obviously, the PPy foam can hardly be applied in the capture of organics and oils from water. To obtain superhydrophobic surfaces of PPy foam, the modification of hydrophobic low-surface-energy material with nano- or micro-sized roughness on the substrate is greatly essential [51–53]. Recently, many absorbents with superhydrophobicity and superoleophilicity, which were achieved by surface modification with low-surface-energy chemicals such as polytetrafluoroethylene [2], tetraethoxysilane [54], polydimethylsiloxane [55], etc., have been issued and successfully used in the capture of organics and oils from water. Choi et al. [6] reported an eco-friendly sugar-templated polydimethylsiloxane sponge promoted potential in environmental applications of oil/water selective separation. Also, Jiang and coworkers [2] studied the superhydrophobicity and superoleophilicity mesh film with a rough surface composed mainly of low-surface-energy PTFE used effectively for the separation of oil and water. Accordingly, to change the surface chemical composition, we treated the proposed PPy foam using a dip-coating technique that provided a low-surface-energy material such as FAS onto the whole surface of porous foam to alter its surface wettability, which resulted in its superhydrophobicity by combination with the microscopic roughness. FAS is a superhydrophobic and moderately superoleophilic material with low-surface energy. As anticipated, upon hydrophobic treatment of FAS, the proposed PPy foam successfully changed from superhydrophilicity to superhydrophobicity, as is made evident by its water CA of 155° (Fig. 2c). Importantly, the proposed FAS-treated PPy foam (FPF) has both properties of superhydrophobicity and superoleophilicity. To demonstrate these excellent properties, we measured the water CA to investigate the surface wettability of FPF. As shown in Fig. 2b, when two water droplets (dyed with methylene blue) were allowed to sit on the rough surface of FPF, the water droplets kept spherical and translucent, which was consistent with the water CA of FPF. In contrast to its high hydrophobic performance, the FPF exhibits good oleophilicity after coating of low-surface-energy material. For instance, when a benzene labeled with Red oil O was put on the surface of FPF upon the commercially white sponge, it was quickly absorbed into the block of FPF and transferred onto the porous surface of sponge (Fig. 2b, inset), resulting in the oil CA of ca. 0° (Fig. 2d). It should be due to that the chain of volatile silicone-contained polyfluorinated alkanes was contacted with the hydrogen atom of PPy and outstretched, taking place of the original hydrogen bond resulting from the effect of H2O, as seen in Fig. 2a.
To investigate the effect of surface composition on the surface wettability of FPF, FT-IR spectra was performed. As shown in Fig. 3, the peaks appeared at 2934, 1634, and 1552 cm−1 were attributed to the results of the =C–H, C=C, and C–C stretching vibrations, respectively. The peak at 1400 cm−1 was assigned to the in-plane C–N stretching vibration [56]. Also, the peaks observed at 906 and 796 cm−1 mainly indicated the characteristic of in-plane C β -H binding deformation and vibration of pyrrole, suggesting that the polymerization combination is attributed to the form of α–α combination between pyrrole rings [57, 58]. Compared with the pure PPy, however, such new peaks at 1240, 1200, and 1148 cm−1 were fully proved to demonstrate the bond of polyfluorinated alkanes (Fig. 3, inset), in good accordance with the literatures [6, 55].
Furthermore, we also use XPS to confirm the effect of FAS modifier on the wettability of PPy foam. From Fig. 4, the peaks appeared at 285.0, 408.02, and 863.70 eV are attributed to the C1s, N1s, and Ni2p, respectively [59, 60]. Obviously, these peaks mentioned above indicated the PPy film coated on the nickel foam. Specially, some characteristic peaks were observed at 689.20, 102.35, and 532.35 eV, which indicated the F1s, Si2p, and O1s and was in good agreement with the previous literatures [61, 62], corresponding to 22.83 at.% fluorine, 5.61 at.% silicon, and 5.92 at.% oxygen, respectively. It should be obviously explained that volatile polyfluorinated silicone-contained alkanes in the form of short FAS chains form a conformal layer on the surface of PPy foam and subsequently crosslink, to lead to the formation of a silicone coating, which is in accordance with the result of FT-IR [24].
Taking advantage of its superhydrophobicity and superoleophilicity as well as its porous feature, the FPF can be a promising candidate for capture of organic solvents and oils from water. As shown in Fig. 5a–c, when the FPF was contacted with a benzene droplet (dyed with Red oil O) on the water surface, it could be quickly absorbed by the FPF without absorbing any water, indicating its excellent selective absorption property. Also, we found that the capture of organics (in this case tetrachloromethane) could be conducted underwater by the FPF (Fig. 5d–f). The fast absorption of organic solvents used should be mainly attributed to the high oleophilic character of the FPF in combination with its porous feature.
Most interestingly, we found that the FPF could possibly be used as a selective membrane (seen in Fig. 6a) for both organic solvents and oils, with great potential for the removal of toxic organic contaminants and oil spills from water. As shown in Fig. 6b, when a mixture of benzene and red O oil was poured onto the surface of superoleophilic FPF, the dyed benzene solution can be quickly absorbed and freely pass through the FPF to achieve high efficiency, illustrating the strong oleophilic performance of FPF. However, the water droplets (dyed with methylene blue for easy observation) were steadily held on the FPF surface (Fig. 6c) and kept spherical (Fig. 6c, inset), implying that the special surface wettability enabled the modified PPy foam for the capture of organics and oils from water with a high absorption selectivity. Meanwhile, as seen in Fig. 6d, e, when the water droplet was squeezed or pulled between two small pieces of FPF, it could also keep spherical, representing the excellent stability of superhydrophobic property of FPF, and revealing this observation can make the FPF the promising candidate as a “mechanical hand” for transferring small quantity of aqueous samples for microsample analysis [59]. Furthermore, the absorption capacity is an important factor for many oleophilic absorbents. For traditional bulk absorbent materials, the absorption efficiency mainly depends on the interaction between the absorbents and the organics [63], and the absorbing capacity is limited. In contract, for the FPF as membrane, the absorption process was continual, which could have potential in the purification of organics separation or oil leakage in water. On the other hand, as a porous-membrane material, the absorption capacity mainly depended on the density of various organics, different from the traditional absorbent materials whose adsorption capacity is limited by their bulk volume. We believe such FPF would be used widely in oil spills cleanup especially for large areas oil leakage on sea or severely polluted water regions.
The recyclability and recoverability of superhydrophobic FPF for capture of organics and oils address key requirements in practical oil cleanup applications, which is an important factor for evaluation of their practical usage performance. In this case, the absorbed organics (in this case benzene) in the FPF can be removed simply by heat treatment or washing. As shown in Fig. 7, the water CA values of the FPF do not change greatly with an average value of 145° (Fig. 7), indicating good recyclability and recoverability due to the tight wrapping of FAS film onto the surface of PPy foam. On the basic point, the FPF would be a desirable material in that it can facilitate the recycling of oil-absorbent materials by allowing for the repeated capture and release of oils and organic solvents. Our study could provide a versatile method for creation of 3D absorbing materials by simple electrodeposition method. Furthermore, compared with those superoleophilic absorbents which were fabricated by complicated process or need multistep procedures, the fabrication of such polymer-coated foam using electrodeposition method and surface modification are simple and easy to be scaled up for large-scale practical applications.
Conclusion
In summary, three-dimensional superhydrophobic material was fabricated by coating of conductive polymers such as PPy onto commercial nickel foam by the electrodepositing method. The superhydrophobic and superoleophilic properties of FPF should be contributed to the formation of nanostructured surface roughness of polymers on the surface of nickel foam by combination with low-surface-energy materials modification. The FPF could be well used as a membrane with high selectivity for the capture of oils and organic solvents from water, which would have great potential in fields of removal of oils and organics from water and is of technological significance.
References
Wu G, Kang HB, Zhang XY, Shao HB, Chu LY, Ruan CJ (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174:1–8
Feng L, Zhang ZY, Mai ZH, Ma YM, Liu BQ, Jiang L, Zhu DB (2004) A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew Chem Int Ed 43:2012–2014
Feng XJ, Jiang L (2006) Design and creation of superwetting/antiwetting surfaces. Adv Mater 18:3063–3078
Xue ZX, Wang ST, Lin L, Chen L, Liu MJ, Feng L, Jiang L (2011) A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv Mater 23:4270–4273
Wen Q, Di JC, Jiang L, Yu JH, Xu RR (2013) Zeolite-coated mesh film for efficient oil–water separation. Chem Sci 4:591–595
Choi SJ, Kwon TH, Im H, Moon DI, Baek DJ, Seol ML, Duarte JP, Choi YK (2011) A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl Mater Interfaces 3:4552–4556
Zhu Q, Chu Y, Wang ZK, Chen N, Lin L, Liu FT, Pan QM (2013) Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J Mater Chem A 1:5386–5393
Calcagnile P, Fragouli D, Bayer IS, Anyfantis GC, Martiradonna L, Cozzoli PD, Cingolani R, Athanassiou A (2012) Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 6:5413–5419
Zhu Q, Pan QM, Liu FT (2011) Facile removal and collection of oils from water surfaces through superhydrophobic and superoleophilic sponges. J Phys Chem C 115:17464–17470
Nguyen DD, Tai NH, Lee SB, Kuo WS (2012) Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci 5:7908–7912
Lin YR, Gregory JE, Colton B, Henry AS (2011) Superhydrophobic functionalized graphene aerogels. ACS Appl Mater Interfaces 3:2200–2203
Choi BG, Park HS (2012) Superhydrophobic graphene/nafion nanohybrid films with hierarchical roughness. J Phys Chem C 116:3207–3211
Zhang L, Zha DA, Du TT, Mei SL, Shi ZJ, Jin ZX (2011) Formation of superhydrophobic microspheres of poly(vinylidene fluoride–hexafluoropropylene)/graphene composite via gelation. Langmuir 27:8943–8949
Dong XC, Chen J, Ma YW, Wang J, Chan-Park MB, Liu XM, Wang LH, Huang W, Chen P (2012) Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water. Chem Commun 48:10660–10662
Cong HP, Ren XC, Wang P, Yu SH (2012) Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 6:2693–2703
Singh E, Chen ZP, Houshmand F, Ren WC, Peles Y, Cheng HM, Koratkar N (2013) Superhydrophobic graphene foams. Small 9:75–80
Jin J, Wang X, Song M (2011) Graphene-based nanostructured hybrid materials for conductive and superhydrophobic functional coatings. J Nanosci Nanotech 11:7715–7722
Zhang XQ, Wan SH, Pu JB, Wang LP, Liu XQ (2011) Highly hydrophobic and adhesive performance of graphene films. J Mater Chem 21:12251–12258
Lee JS, Yoon JC, Jang JH (2013) A route towards superhydrophobic graphene surfaces: surface-treated reduced graphene oxide spheres. J Mater Chem A 1:7312–7315
Chen ZX, Dong L, Yang D, Lu HB (2013) Superhydrophobic graphene-based materials: surface construction and functional applications. Adv Mater 25:5352–5359
Han JT, Kim JS, Kim SH, Lim HS, Jeong HJ, Jeong SY, Lee GW (2010) Nanocarbon-induced rapid transformation of polymer surfaces into superhydrophobic surfaces. ACS Appl Mater Interfaces 2:3378–3383
Gui XC, Wei JQ, Wang KL, Cao AY, Zhu HW, Jia Y, Shu QK, Wu DH (2010) Carbon nanotube sponges. Adv Mater 22:617–621
Lee CH, Johnson N, Drelich J, Yap YK (2011) The performance of superhydrophobic and superoleophilic carbon nanotube meshes in water–oil filtration. Carbon 49:669–676
Yuan JK, Liu XG, Akbulut O, Hu JQ, Suib SL, Kong J, Stellaaai F (2008) Superwetting nanowire membranes for selective absorption. Nat Nanotechnol 3:332–336
Zhang JP, Seeger S (2011) Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv Funct Mater 21:4699–4704
Darmanin T, Nicolas M, Guittard F (2008) Electrodeposited polymer films with both superhydrophobicity and superoleophilicity. Phys Chem Chem Phys 10:4322–4326
Yang J, Zhang ZZ, Xu XH, Zhu XT, Men XH, Zhou XY (2012) Superhydrophilic–superoleophobic coatings. J Mater Chem 22:2834–2837
Crick CR, Gibbins JA, Parkin IP (2013) Superhydrophobic polymer-coated copper-mesh membranes for highly efficient oil–water separation. J Mater Chem A 1:5943–5948
Cao YZ, Zhang XY, Tao L, Li K, Xue ZX, Feng L, Wei Y (2013) Mussel-inspired chemistry and michael addition reaction for efficient oil/water separation. ACS Appl Mater Interfaces 5:4438–4442
Basu BBJ, Paranthaman AK (2009) A simple method for the preparation of superhydrophobic PVDF–HMFS hybrid composite coatings. Appl Surf Sci 255:4479–4483
Feng L, Song YL, Zhai J, Liu BQ, Xu J, Jiang L, Zhu DB (2003) Creation of a superhydrophobic surface from an amphiphilic polymer. Angew Chem Int Ed 115:824–826
Hayase G, Kanamori K, Fukuchi M, Kaji H, Nakanishi K (2013) Facile synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. Angew Chem Int Ed 52:1986–1989
Ono T, Sugimoto T, Shinkai S, Sada K (2007) Lipophilic polyelectrolyte gels as super-absorbent polymers for nonpolar organic solvents. Nat Mater 6:429–433
Sonmez HB, Wudl F (2005) Cross-linked poly(orthocarbonate)s as organic solvent sorbents. Macromolecules 38:1623–1626
Su C (2009) Highly hydrophobic and oleophilic foam for selective absorption. Appl Surf Sci 256:1413–1418
Zhang X, Li Z, Liu K, Jiang L (2013) Bioinspired multifunctional foam with self-cleaning and oil/water separation. Adv Funct Mater 23:2881–2886
Liu H, Liu Z, Yang M, He Q (2013) Surperhydrophobic polyurethane foam modified by graphene oxide. J Appl Polym Sci 130:3530–3536
Li A, Sun HX, Tan DZ, Fan WJ, Wen SH, Qing XJ, Li GX, Li SY, Deng WQ (2011) Superhydrophobic conjugated microporous polymers for separation and adsorption. Energy Environ Sci 4:2062–2065
Sun HX, Li A, Zhu ZQ, Liang WD, Zhao XH, La PQ, Deng WQ (2013) Superhydrophobic activated carbon-coated sponges for separation and absorption. ChemSusChem 6:1057–1062
Fan ZL, Qin XJ, Sun HX, Zhu ZQ, Pei CJ, Liang WD, Bao XM, An J, La PQ, Li A, Deng WQ (2013) Superhydrophobic mesoporous graphene for separation and absorption. ChemPlusChem 78:1282–1287
Sun HX, Li A, Qin XJ, Zhu ZQ, Liang WD, La PQ, Deng WQ (2013) Three-dimensional superwetting mesh film based on graphene assembly for liquid transportation and selective absorption. ChemSusChem 6:2377–2381
Arbatan T, Fang XY, Shen W (2011) Superhydrophobic and oleophilic calcium carbonate powder as a selective oil sorbent with potential use in oil spill clean-ups. Chem Eng J 166:787–791
Su CH, Xu YQ, Zhang W, Liu Y, Li J (2012) Porous ceramic membrane with superhydrophobic and superoleophilic surface for reclaiming oil from oily water. Appl Surf Sci 258:2319–2323
Lin JJ, Chu CC, Chiang ML, Tsai WC (2006) Manipulating assemblies of high-aspect-ratio clays and fatty amine salts to form surfaces exhibiting a lotus effect. Adv Mater 18:3248–3252
Zhong WB, Liu SM, Chen XH, Wang YX, Yang WT (2006) High-yield synthesis of superhydrophilic polypyrrole nanowire networks. Macromolecules 39:3224–3230
Xu LB, Chen W, Mulchandani A, Yan YS (2005) Reversible conversion of conducting polymer films from superhydrophobic to superhydrophilic. Angew Chem Int Ed 44:6009–6012
Chang JH, Hunter IW (2011) A superhydrophobic to superhydrophilic in situ wettability switch of microstructured polypyrrole surfaces. Macromol Rapid Commun 32:718–723
Li M, Wei ZX, Jiang L (2008) Polypyrrole nanofiber arrays synthesized by a biphasic electrochemical strategy. J Mater Chem 18:2276–2280
Jiang L, Zhao Y, Zhai J (2004) A lotus-leaf-like superhydrophobic surface: a porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew Chem Int Ed 116:4438–4441
Feng L, Li S, Li Y, Li H, Zhang L, Zhai J, Song Y, Liu B, Jiang L, Zhu D (2002) Super-hydrophobic surfaces: from natural to artificial. Adv Mater 14:1857–1860
Gao L, McCarthy TJ (2006) A perfectly hydrophobic surface (θA/θR = 180/180). J Am Chem Soc 128:9052–9053
Lau KKS, Bico J, Teo KBK, Chhowalla M, Amaratunga GAJ, Milne WI, McKinley GH, Gleason KK (2003) Superhydrophobic carbon nanotube Forests. Nano Lett 3:1701–1705
Motornov M, Sheparovych R, Lupitskyy R, MacWilliams E, Minko S (2008) Superhydrophobic surfaces generated from water-borne dispersions of hierarchically assembled nanoparticles coated with a reversibly switchable shell. Adv Mater 20:200–205
Yang H, Pi PH, Cai ZQ, Wen XF, Wang XB, Cheng J, Yang ZR (2010) Facile preparation of super-hydrophobic and super-oleophilic silica film on stainless steel mesh via sol-gel process. Appl Surf Sci 256:4095–4102
Cui JF, Bao XM, Sun HX, An J, Guo JH, Yang BP, Li A (2014) Preparation of superhydrophobic surfaces by cauliflower-like polyaniline. J Appl Poly Sci. doi:10.1002/app.39767
Tian B, Zerbi G (1990) Lattice dynamics and vibrational spectra of polypyrrole. J Chem Phys 92:3886–3892
Münstedt H (1986) Properties of polypyrroles treated with base and acid. Polymer 27:899–904
Marco ADP, Waltman RJ, Diaz AF, Bargon J (1985) An electrically conductive plastic composite derived from polypyrrole and poly(vinyl chloride). J Poly Sci Chem Ed 23:1687–1689
He YJ, Lu JH (2007) Synthesis of polyaniline nanostructures with controlled morphology by a two-phase strategy. React Funct Polym 67:476–480
Chen SG, Chen Y, Lei YH, Yin YS (2009) Novel strategy in enhancing stability and corrosion resistance for hydrophobic functional films on copper surfaces. Electrochem Commun 11:1675–1679
Hong R, Pan T, Qian J, Li H (2006) Synthesis and surface modification of ZnO nanoparticles. Chem Eng J 119:71–81
Shin H, Kim KK, Benayad A, Yoon S, Park HK, Jung I, Jin MH, Jeong H, Kim JM, Choi J, Lee YH (2009) Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv Funct Mater 19:1987–1992
Chen H, Wang A (2007) Kinetic and isothermal studies of lead ion adsorption onto palygorskite clay. J Colloid Interface Sci 307:309–316
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (Grant Nos. 51263012 and 51262019) and Gansu Provincial Science Fund for Distinguished Young Scholars (Grant No. 1308RJDA012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, J., Sun, H., Cui, J. et al. Surface modification of polypyrrole-coated foam for the capture of organic solvents and oils. J Mater Sci 49, 4576–4582 (2014). https://doi.org/10.1007/s10853-014-8157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8157-8