Abstract
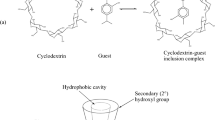
Cyclodextrins have been extensively used for inclusion of various drugs for improvement of pharmaceutical characteristics of diverse nature. Numerous derivatives of cyclodextrins are currently available and are being subjected to continuous investigations. On the other hand, urea was previously being used as host for inclusion of straight chain linear compounds. However, development of a modified technique has opened flood gates for inclusion of non-linear drugs containing cyclic moieties. Cis-retinoic acid, nicorandil, vitamin E, amiloride, glipizide, enalapril maleate, simvastatin, lafutidine and ezetimbe are some of the drugs which have already been investigated for improvement of pharmaceutical characteristics. These characteristics include improvement of stability, content uniformity, dissolution profile and rheological behavior. Recently, pesticide-fertilizer combination has also been reported to serve the dual purpose of pesticide cum fertilizer and for improvement in safe handling and formulation characteristics. All FDA approved cyclodextrins are safe chemicals for human use within permissible limits. Urea is a component of normal physiological processes of body in human beings and other mammals. Urea is far cheaper when compared to cyclodextrins. Studies reveal that despite unique characteristics of urea like high solubility, non-toxicity, stability, inexpensiveness, biodegradability and easy availability, the use of urea as a host for inclusion of drugs has been grossly overlooked by researchers. Immense potential of urea as a host for inclusion of drugs need to be explored for improvement of pharmaceutical characteristics. The relative use of both cyclodextrins and urea as hosts for inclusion of drugs has been briefly reviewed in the present article.



Similar content being viewed by others
References
Lehn, J.M.: Towards complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 99, 4763–4768 (2002)
Findlay, R.A.: Adductive crystallization. In: Schoen, H.M., Mcketta, J.J. (eds.) Interscience Library of Chemical Engineering and Processing. New Chemical Engineering Separation Techniques, vol. 1, 257–318. Interscience Publishers, New York (1962)
Bhatnagar, V.M.: Clathrate compounds of urea and thiourea. J. Struc. Chem. 8(3), 513–529 (1968)
Frank, S.G.: Inclusion compounds. J. Pharm. Sci. 64, 1585–1604 (1975)
Sinko, P.J. (ed.): Martin’s Physical Pharmacy and Pharmaceutical Sciences. 6th edn, pp. 197–222. Wolters Kluwer, New Delhi (2005)
Dyadin, Y.A., Terekhova, I.S.: Classical description of inclusion compounds. In: Atwood, J.L., Steed, J.W. (eds.) Encyclopedia of Supramolecular Chemistry, vol. 2, pp. 253–260. Marcel Dekker, New York (2004)
Powell, H.M.: The structure of molecular compounds. Part IV. Clathrate compounds. J. Chem. Soc. 61–72 (1948). doi:10.1039/JR9480000061
Schlenk, W.: Urea addition of aliphatic compounds. Ann. Chem. 565, 204–240 (1949)
Weber, E.: Clathrate chemistry today-some problems and reflections. Topics Curr. Chem. 140, 3–20 (1989)
Madan, A.K., Thakral, S.: Urea as an adductor for branched drug molecules. In: Fitzpatrick, D.W., Ulrich, H.J. (eds.) Macrocyclic Chemistry: New Research Developments. Chapter 18, pp. 469–498. Nova Science Publishers Inc., New York (2010)
Bishop, R., Dance, I.G.: New type of helical inclusion compounds. Topics Curr. Chem. 149, 139–188 (1988)
Weber, E., Josel, H.P.: A proposal for the classification and nomenclature of host-guest type compounds. J. Incl. Phenom. 1, 79–85 (1983)
Cram, D.J.: Host-guest complex. In: Boschke, E.L. (ed.) Topics in Current Chemistry, vol. I-III, pp. 1982–1984. Springer Verlag, Berlin (1986)
Steed, J.W., Atwood, J.L. (eds.): Supramolecular Chemistry, pp. 1–6. Wiley, Chichester (2000)
Powell, H.M.: Introduction. In: Atwood, J.W., Davis, J.E.D., MacNicol, D.D. (eds.) Inclusion compounds, vol. 1, pp. 1–28. Academic Press, London (1984)
Harris, K.D.M.: Meldola Lecture: understanding properties of urea and thiourea inclusion compounds. Chem. Soc. Rev. 76, 279–289 (1997)
George, A.R., Harris, K.D.M.: Representing and understanding geometric features of one-dimensional tunnel structures in solid inclusion compounds. J. Mol. Graphics. 13, 138–141 (1995)
Hagan, M.M. (ed.): Clathrate inclusion compounds, pp. 30–75. Reinhold Publishing Corporation, California (1962)
Rapson, W.S., Saunder, D.H., Stewart, E.T.: Molecular compound formation in the polyphenyl series; some compounds formed by 4:4′-dinitrodiphenyl. J. Chem. Soc. (1946). doi:10.1039/JR9460001110
Rundle, R.E., Baldwin, R.R.: The configuration of starch and the starch-iodine complex: The dichroism of flow of starch-iodine solutions. J. Am. Chem. Soc. 65, 554–558 (1943)
Lahr, P.H., Williams, H.L.: Properties of some rare gas clathrate compounds. J. Phys. Chem. 63, 1432–1434 (1959)
Williams, D.J., Lawton, D.: Deviations from C3 symmetry of the tri-o-thymodite molecule in different crystalline environments: X-ray determinations of the unsolvated form and of typical cavity and channel inclusion compounds. Tetrahedron Lett. 16, 111–114 (1975)
Flippen, J.L., Karle, J.: Heptanol as a guest molecule in Dianin’s compound. J. Phys. Chem. 75, 3566–3567 (1971)
D’Souza, V.T., Lipkowitz, K.B.: Cyclodextrins: introduction. Chem. Rev. 98, 1741–1742 (1998)
Nicolini, C., Ramoa, F., Ribeiro, E.J., Duckstein, L. (eds.): Zeolites: Science and Technology. Springer, Netherland (1987)
Makha, M., Raston, C.L., Sobolev, A.N., Barbour, L.J., Turner, P.: Endo- versus exo-cavity interplay of p-benzylcalix[4]arene with spheroidal molecules. Cryst. Eng. Comm. 8, 306–308 (2006). doi: 10.1039/B600550K
Lin, R.L., Fang, G.S., Sun, W.Q., Liu, J.X.: Aniline-containing guests recognized by α,α’,δ,δ’-tetramethyl-cucurbit[6]uril host. Sci.Rep. 6, 39057–39060 (2016). doi:10.1038/srep39057
Hardie, M.J., Johnson, J.A., Raston, C.L., Webb, H.R.: Cooperative hydrogen bonding and yttrium (III) complexation in the assembly of molecular capsules. Chem. Commun. 10, 849–850 (2000). doi:10.1039/A910256F
Tarantula, O., Hill, P.H., Khan, N.S., Carroll, P.J., Dmochowski, I.J.: Crystallographic observation of ‘induced fit’ in a cryptophane host–guest model system. Nat. Commun. 1, 148–149 (2010). doi:10.1038/ncomms1151
Vaijayanthimala, G., Krishan, V., Mandal, S.K.: Cyclic porphyrin dimers as hosts for coordinating ligands. J.Chem. Sci. 120(1), 115–129 (2008)
Hu, x.B., Chen, Z., Chen, L., Zhang, L., Hou, J.L., Li, Z.T.: Pillar[n]arenes (n = 8–10) with two cavities: synthesis, structures and complexing properties. Chem. Commun. 48, 10999–11001 (2012). doi:10.1039/C2CC36027F
Allen, L.V., Popovich, N.G., Ansel, H.C. (eds.): Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. 8th edn, pp. 93–140. Lippincott Williams and Wilkins, Philadelphia (2005)
Huang, Y., Dai, W.G.: Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharmaceutica Sinica B. 4(1), 18–25 (2014)
Dhirendra, K.S., Lewis, S., Udupa, N., Atin, K.: Solid dispersions: a review. Pak. J. Pharm. Sci. 22(2), 234–246 (2009)
Ingle, U.S., Gaikwad, P.D., Bankar, V.H., Pawar, S.P.: A review on solid dispersion: a dissolution enhancement technique. IJRAP 2(3), 751–757 (2011)
Baghel, S., Cathcart, H., O’Reilly, N.J.: Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 105, 2527–2544 (2016)
Szejtli, J.: Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 7, 575–587 (1997)
Huh, K.M., Lee, S.C., Ooya, J., Park, K.: Polymeric delivery system for poorly soluble drugs. In: Swarbrick, J. (ed.) Encyclopedia Pharmaceutical Technology, vol. 5, 3rd edn, pp. 2912–2917. Informa HealthCare, New York (2007)
Yoshioka, S., Stella, V.J. (eds.): Stability of Drugs and Dosage Forms. pp. 106–107. Springer, Netherlands (2006)
Marangoci, N., Mares, M., Silion, M., Fifere, A., Varganici, C., Nicolescu, A., Deleanu, C., Coroaba, A., Pinteala, M., Simionescu, B.: Inclusion complex of a new propiconazole derivative with β-cyclodextrin: NMR, ESI–MS and preliminary pharmacological studies. Results Pharma. Sci. 1(1), 27–37 (2011). doi:10.1016/j.rinphs.2011.07.001
Gidwani, B., Vyas, A.: A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015 (2015). doi:10.1155/2015/198268
Szejtli, J., Osa, T. (eds.): Cyclodextrins. In: Comprehensive Supramolecular Chemistry, vol. 3, Pergamon, Oxford (1996)
Loftsson, T., Brewster, M.E., Masson, M.: Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2(4), 1–15 (2004)
Loftsson, T., Masson, M., Brewster, M.E.: Self association of cyclodextrins and cyclodextrin complexes. J. Pharm. Sci. 93, 1091–1099 (2004)
Das, S., Rajabalaya, R., David, S., Gani, N., Khanam, J., Nanda, A.: Cyclodextrins-the molecular structure. RJPBCS 4(2), pp. 1694–1720 (2013)
Katageri, A.R., Sheikh, M.A.: Cyclodextrin: a gift to pharmaceutical world review. IRJP 3(1), 52–56 (2012)
Anjana, M., Nair, S.C., Joseph, J.: An updated review of cyclodextrins–An enabling technology for challenging pharmaceutical formulations. Int. J. Pharm. Pharm. Sci. 5(3), 54–58 (2013)
Shimpi, S., Chauhan, B., Shimpi, P.: Cyclodextrins: application in different routes of drug administration. Acta Pharm. 55, 139–156 (2005)
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023–1035 (2004)
Valle, E.M.: Cyclodextrins and their uses: A review. Process Biochem. 39(9), 1033–1046 (2004). doi:10.1016/S0032-9592(03)00258-9
Crini, G.: Review: a history of cyclodextrins. Chem. Rev. 114(21), 10940–10975 (2014). doi:10.1021/cr500081p
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85(10), 1017–1025 (1996)
Carrier, R.L., Miller, L.A., Ahmed, I.: The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Rel. 123, 78–99 (2007)
Uekema, K., Fujinaga, T., Hirayama, F.: Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J. Pharm. Sci. 72, 1338–1341 (1983)
Cui, L., Zhang, Z., Sun, E., Jia, X., Qian, Q.: Effect of β-cyclodextrin complexation on solubility and enzymatic hydrolysis rate of icariin. J. Nat. Sci. Biol. Med. 4(1), 201–206 (2013). doi:10.4103/0976-9668.107291
Singhla, A.K., Garg, A., Aggarwal, D.: Paclitaxel and its formulations. Int. J. Pharm. 235, 179–192 (2002)
Garbow, J.R., Likos, J.J., Schroeder, S.A.: Structure, dynamics, and stability of beta-cyclodextrin inclusion complexes of aspartame and neotame. J. Agric. Food Chem. 49(4), 2053–2060 (2001)
Teixeria, L.R., Sinisterra, R.D., Vieria, R.P., Scarlatelli-Lima, A., Moraes, M.F.D., Doretto, M.C., Denadai, A.M., Beraldo, H.: An inclusion compound of anticonvulsant sodium valproate into α-cyclodextrin: physic-chemical characterization. J. Incl. Phenom. Macrocycl. Chem. 54, 133–138 (2006)
Loftsson, T., Peterson, D.S.: Cyclodextrin solubilization of ETH-615, a zwitterionic drug. Drug Dev. Ind. Pharm. 24, 365–370 (1998)
Ioele, G., De Luca, M., Ragno, G.: Photostability of barnidipine in combined cyclodextrin-in-liposome matrices. Future Med. Chem. 6(1), 35–43 (2014). doi:10.4155/fmc.13.187
Rivas-Granizo, P.E., Giorgretti, L., Ferraz, H.G.: Photostability of loratadine inclusion complexes with natural cyclodextrins. Int. J. Photoenergy (2015). doi:10.1155/2015/583052
Pomponio, R., Gotti, R., Fiori, J., Cavrini, V., Mura, P., Cirri, M., Maestrelli, F.: Photostability studies on nicardipine-cyclodextrin complexes by capillary electrophoresis. J. Pharm. Biomed. Anal. 35(2), 267–275 (2004)
Cwiertnia, B., Hladon, T., Stobiecki, M.: Stability of diclofenac sodium in the inclusion complex in the beta cyclodextrin in the solid state. J. Pharm. Pharmacol. 51, 1213–1218 (1999)
Ndlebe, V.J., Brown, M.E., Glass, B.D.: The thermal stability of triprolidine hydrochloride and its mixture with cyclodextrin and glucose. J. Therm. Analy. Calori. 77(2), 445–457 (2004)
Popescu, C., Manda, P., Juluri, A., Janga, K.Y., Cidda, M., Murthy, S.N.: Enhanced dissolution efficiency of zalephon solid dispersions via modified β-cyclodextrins molecular inclusion complexes. J. Pharm. Pharm. Sci. 1(1), 1–10 (2015)
Yoganada, R., Chowdary, K.P.R.: Enhancement of solubility, dissolution rate and bioavailability of efavirenz by cyclodextrins and solutol HS15—A factorial study. Int. J. Drug. Dev. Res. 5(1), 135–142 (2013)
Chowdary, K.P.R., Reddy, M.V.: Formulation development studies on enhancement of solubility and dissolution rate of etoricoxib by cyclodextrin complexation. Asian J. Chem. 23(4), 1445–1448 (2011)
Uekama, K., Ikegami, K., Wang, Z.: Inhibitory effect of 2-hydroxypropyl-β-cyclodextrin on crystal growth of nifedipine during storage: superior dissolution and oral bioavailability compared with polyvinylpyrrolidone K-30. J. Pharm. Pharmacol. 44, 73–78 (1992)
Aggarwal, S., Singh, P.N., Mishra, B.: Studies on solubility and hypoglycemic activity of gliclazide β-cyclodextrin-hydroxypropylmethylcellulose complexes. Pharmazie. 57, 191–193 (2002)
Latrofa, A., Trapani, G., Franco, M.: Complexation of phenytoin with some hydrophilic cyclodextrins: effect on aqueous solubility, dissolution rate and anti-convulsant activity in mice. Eur. J. Pharm. Biopharm. 52, 65–73 (2001)
Ahn, H.J., Kim, K.M., Choi, S.J., Kim, C.K.: Effects of cyclodextrin derivatives on bioavailability of ketoprofen. Drug Dev. Ind. Pharm. 23, 397–401 (1997)
Londhe, V., Nagarsenker, M.: Comparison between hydroxypropyl-β-cyclodextrin and polyvinylpyrrolidine as carriers for carbamazepine solid dispersions. Indian J. Pharm. Sci. 61, 237–240 (1999)
Jayachandra Babu, R., Pandit, J.K.: Effect of aging on the dissolution stability of glibenclamide/ β-cyclodextrin complex. Drug Dev. Ind. Pharm. 25(11), 1215–1219 (1995). doi:10.1081/DDC-100102291
Cavallari, C., Abertini, B., Rodriguez, M.L.G., Rodriguez, L.: Improved dissolution behavior of steam granulated piroxicam. Eur. J. Pharm. Biopharm. 54, 65–73 (2002)
Sanghavi, N.M., Choudhari, K.B., Matharu, R.S., Viswanathan, L.: Inclusion complexation of lorazepam with β-cyclodextrin. Drug Dev. Ind. Pharm. 19, 701–712 (1993)
Dhanraju, M.D., Santil, K., Baskaran, T., Moorthy, M.S.R.: Enhancement of bioavailability of griseofulvin by its complexation with beta-cyclodextrin. Drug. Dev. Ind. Pharm. 24, 583–587 (1998)
Bettinetti, G., Gazzaniga, A., Mura, P., Giordano, F., Setti, M.: Thermal behavior and dissolution properties of naproxen in combinations with chemically modified beta-cyclodextrins. Drug Dev. Ind. Pharm. 18, 39–53 (1992)
Meilcarek, J., Czernielewaska, A., Czarczynska, B.: Inclusion complexes of felodipine and amlodipine with methyl-β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 54, 17–21 (2006)
Kaukonen, A.M., Lennernas, H., Mannermaa, J.P.: Water soluble β-cyclodextrin in paediatric oral solutions of spiranolactone: preclinical evaluation of spiranolactone bioavailability from solutions of β-cyclodextrin derivatives in rats. J Pharm. Pharmacol. 50, 611–619 (1998)
Jain, A.C., Adeyeye, M.C.: Hygroscopicity, phase solubility and dissolution of various substituted sulfobutylether beta-cyclodextrins (SBE) and danzol-SBE inclusion complexes. Int. J. Pharm. 212, 177–186 (2001)
Arias, M.J., Moyano, J.R., Munoz, P., Gines, J.M., Justo, A., Giordano, F.: Study of omeprazole-gamma-cyclodextrin complexation in the solid state. Drug Dev. Ind. Pharm. 26, 253–259 (2000)
Medlicott, N.J., Foster, K.A., Audis, K.L., Gupta, S., Stella, V.J.: Comparison of effects of potential parenteral vehicles for poorly water soluble anticancer drugs on cultured endothelial cells. J. Pharm. Sci. 87, 1138–1143 (1998)
Funasaki, N., Kawaguchi, R., Hada, S., Neya, S.: Ultraviolet spectroscopic estimation of microenvironments and bitter tastes of oxyphenonium bromide in cyclodextrin solutions. J. Pharm. Sci. 88(8), 759–762 (1999)
Jagdale, S.C., Gawali, V.U., Kuchekar, B.S., Chabukswar, A.R.: Formulation and in vitro evaluation of taste-masked oro-dispersible dosage form of diltiazem hydrochloride. Braz. J. Pharm. Sci. 47(4), 907–916 (2011)
Patel, A.R., Vavia, P.R.: Preparation and evaluation of taste masked famotidine formulation using drug/ β-cyclodextrin/ polymer ternary complexation approach. AAPS Pharm. Sci. Tech. 9(2), 544–550 (2008)
Serni, U.: Rheumatic diseases-clinical experience with piroxicam beta-cyclodextrin. Eur. J. Rheumatol. Inflamm. 12, 47–54 (1993)
Blanchard, J., Ugwu, S.O., Bhardwaj, R., Dorr, R.T.: Development and testing of improved phenytoin using 2-hydroxypropyl-betacyclodextrin. Pharm. Dev. Technol. 5, 333–338 (2000)
Vafaei, S.Y., Dinarvand, R., Esmaeili, M., Mahjub, R., Toliyat, T.: Controlled-release drug delivery system based on fluocinolone acetonide-cyclodextrin inclusion complex incorporated in multivesicular liposomes. Pharm. Dev. Technol. 26, 1–7 (2014)
Nicolazzi, C., Venard, V., Le Faou, A., Finance, C.: In vitro antiviral activity of the ganciclovir complexed with beta-cyclodextrin on human cytomegalovirus strains. Antiviral Res. 54, 121–127 (2002)
Bhardwaj, R., Dorr, R.T., Blanchard, J.: Approaches to reducing toxicity of parenteral anticancer drug formulations using cyclodextrins. PDA J. Pharm. Sci. Technol. 54(3), 233–239 (2000)
Li, J., Guo, Y., Zografi, G.: The solid state stability of amorphous quinapril in the presence of beta-cyclodextrins. J. Pharm. Sci. 91, 229–243 (2002)
Zong, Z., Desai, S.D., Kaushal, A.M., Barich, D.H., Huang, H.S., Munson, E.J., Suryanarayanan, R., Kirsch, L.E.: The stabilizing effect of moisture on the solid-state degradation of gabapentin. AAPS Pharm. Sci. Tech. 12(3), 924–931 (2011). doi:10.1208/s12249-011-9652-8
Szente, L., Szejtli, J. (eds.): Flavor Encapsulation. ACS Symposium Series. vol. 370, pp. 148–157. American Chemical Society, Hungary (1988). doi:10.1021/bk-1988-0370.ch016
Del Valle, M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Chittiteeranon, P., Soontarus, S., Pongsawasdi, P.: Preparation and characterization of inclusion complexes containing fixolide, a synthetic musk fragrance and cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 57, 69–73 (2007)
Jarho, P., Van der Velde, D., Stella, V.J.: Cyclodextrin-catalyzed deacetylation of spiranolactone is pH and cyclodextrin dependent. J. Pharm. Sci. 89, 241–249 (2000)
Babu, J.R., Pandit, J.K.: Enhancement of dissolution rate and hypoglycemic activity of glibenclamide with β-cyclodextrin. STP Pharma. Sci. 5, 196–201 (1995)
Narisawa, S., Stella, V.J.: Increased shelf-life of fosphenytoin: Solubilization of a degradant, phenytoin, through complexation with (SBE) 7 m-beta-CD. J. Pharm. Sci. 87(8), 926–930 (1998)
Yano, H., Hirayama, F., Kamada, M., Arima, H., Uekama, K.: Colon specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J. Control Release. 79, 103–112 (2002)
Minami, K., Hirayama, F., Uekama, K.: Colon-specific drug delivery based on a cyclodextrin prodrug : release behavior of biphenyl acetic acid from its cyclodextrin conjugates in rat intestinal tracts after oral administration. J. Pharm. Sci. 87(6), 715–720 (1998)
Zou, M.J., Cheng, G., Okamoto, H., Hao, X.H., An, F., Cui, F.D., Danjo, K.: Colon-specific drug delivery systems based on cyclodextrin prodrugs: In vivo evaluation of 5-aminosalicylic acid from its cyclodextrin conjugates. World J. Gastroenterol. 11(47), 7457–7460 (2005). doi:10.3748/wjg.v11.i47.7457
Jarvinen, T., Jarvinen, K., Urtti, A., Thompson, D., Stella, V.J.: Sulfobutyl ether β-cyclodextrin (SBE-β-CD) in eye drops improves the tolerability of a topically applied pilocarpine prodrug in rabbits. J. Ocular Pharmacol. Ther. 11, 95–106 (1995)
Jarho, P., Jarvinen, K., Urtti, A., Stella, V.J., Jarvinen, T.: Modified β-cyclodextrin (SBE7-β-CD) with viscous vehicle improves the ocular delivery and tolerability of pilocarpine prodrug in rabbits. J. Pharm. Pharmacol. 48, 263–269 (1996)
Davies, N.M., Wang, G., Tucker, I.G.: Evaluation of a hydrocortisone/hydroxypropyl-β-cyclodextrin solution for ocular drug delivery. Int. J. Pharm. 156, 201–209 (1997)
Tirucherai, G.S., Mitra, A.K.: Effect of hydroxypropyl beta-cyclodextrin complexion on aqueous solubility, stability and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS PharmSciTech. 4(3), 124–135 (2003)
Hermens, W.A.J.J., Deurloo, M.J.M., Romeijn, S.G., Verhoef, J.C., Merkus, F.W.H.M.: Nasal absorption enhancement of 17-β-oestradiol by dimethyl-β-cyclodextrin in rabbits and rats. Pharm. Res. 7, 500–503 (1990)
Loftsson, T., Guomundsdottir, H., Sigurjonsdottir, J.F.., Sigurosson, H.H., Sigfusson, S.D., Masson, M., Stefannsson, E.: Cyclodextrin solubilization of benzodiazepines: formulation of midazolam nasal spray. Int. J. Pharm. 212, 29–40 (2001)
Matsubara, K., Abe, K., Irie, T., Uekama, K.: Improvement of nasal bioavailability of luteinizing hormone-releasing hormone agonist, buserelin, by cyclodextrin derivatives in rats. J. Pharm. Sci. 84, 1295–1300 (1995)
Lin, S.Z., Wouessidjewe, D., Poelman, M.C., Duchene, D.: In-vivo evaluation of indomethacin/ cyclodextrin complexes gastrointestinal tolerance and dermal anti-inflammatory activity. Int. J. Pharm. 106, 63–67 (1994)
Loftsson, T., Sigurardottir, A.M.: The effect of polyvinyl pyrrolidone and hydroxypropyl methyl cellulose on hydroxypropyl-β-cyclodextrin complexation of hydrocortisone and its permeability through hairless mouse skin. Eur. J. Pharm. Sci. 2, 297–301 (1994)
Loftsson, T., Fririksdottir, H., Thorisdottir, S., Stefansson, E.: The effect of hydroxypropyl methyl cellulose on the release of dexamethasone from aqueous 2-hydroxypropyl-β-cyclodextrin formulations. Int. J. Pharm. 104, 181–184 (1994)
Tiwari, G., Tiwari, R., Rai, A.K.: Cyclodextrins in delivery systems: applications. J. Pharm. Bioallied Sci. 2(2), 72–79 (2010). doi:10.4103/0975-7406.67003
Agrawal, R., Gupta, V.: Cyclodextrins-a review on pharmaceutical application for drug delivery. IJPER 2(1), 95–112 (2012)
Kang, K., Huang, W., Fu, Y., Chen, L., hu, J., Ren, Y.: Pyridine-incorporated cyclo[6]aramide for recognition of urea and its derivatives with two different binding modes. Supramol. Chem. (2011). doi:10.1080/10610278.2017.1282614
Lascaux, A., Leener, G.D., Fusaro, L., Topic, F., Rissanen, K., Luhmer, M., Jabin, I.: Selective recognition of neutral guests in an aqueous medium by a biomimetic calix[6]cryptamide receptor. Org. Biomol. Chem. 14, 738–746 (2016). doi:10.1039/C5OB02067K
Leener, G.D., Moerkerke, S., Lavendomme, R., Reinaud, O., Jab, I.: Calix[6]azacryptand-based receptors. In: Neri, P., Sessler, J.L., Wang, M.X. (eds.) Calixarenes and Beyond, pp. 113–140. Springer International Publishing, Switzerland (2016). doi:10.1007/978-3-319-31867-7_6
Maria, D.S., Farran, M.A., Garcia, M.A., Pinilla, E., Torres, M.R., Elguero, J., Claramunt, R.M.: Synthetic hosts for molecular recognition of ureas. J. Org. Chem. 76(16), 6780–6788 (2011). doi:10.1021/jo201191x
Bengen, M.F.: Urea channel inclusion compounds. German Patent Application OZ123438 Mar 18 (1940)
Abu-Nasr, A.M., Potts, W.M., Holman, R.T.: Highly unsaturated fatty acids. II. Fractionation by urea inclusion compounds. J. Am. Oil Chemists Soc. 31, 16–20 (1954)
Hayes, D.: Urea inclusion compound formation. Inform. 13, 781–801 (2002)
Schlenk, H., Holman, R.H.: Separation and stabilization of fatty acids by urea complexes. J. Am. Chem. Soc. 72, 5001–5004 (1950)
Karr, C. Jr.: Separation process utilizing urea paraffin chromatography. United States Patent. 2,912,426 (1955)
Karr, C., Comberiati, J.R.: The analysis of straight-chain aliphatics by urea partition chromatography and gas-solid chromatography. J. Chromatog. 18, 394–397 (1965)
Oswald, A.A., Chen, F.J., Espino, R.L., Peng, K.L.: Multistep process for the manufacture of novel polyolefin lubricants. United States Patent 5017279 (1991)
Gupta, A. A., Swamy, K. K., Prakash, S., Rai, M. M., Bhatnagar, A. K.: Process for recovery of solid and reusable urea from the urea adduction process. United States Patent 5847209 (1998)
Hollingsworth, M.D., Harris, K.D.M.: Urea inclusion compounds. In: Atwood, J.L. (ed.) Comprehensive Supramolecular Chemistry, Chapter 4, pp. 192–234. Interscience Pub., New York (1996)
Madan, A.K., Grover, P.D.: A process for preparation of urea based inclusion compounds of vitamin A esters. Indian Patent 180627 dated 20/01/1993 (1993)
Madan, A.K., Bajaj, V.: A process for preparation of urea based inclusion compounds of vitamin E and its esters. Indian Patent 182620 dated 24/10/ 1994 (1994)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59(7), 645–666 (2007). doi:10.1016/j.addr.2007.05.012
Ueda, H., Wakamiya, T., Endo, H., Nagase, H., Tomono, K., Nagai, T.: Interaction of cyclomaltononaose (delta-CD) with several drugs. Drug Dev. Ind. Pharm. 25, 951–955 (1999)
Szaniszlo, N., Fenyvesi, E., Balla, J.: Structure-stability study of cyclodextrin complexes with selected volatile hydrocarbon contaminants of soils J. Incl. Phenom. Macrocycl. Chem. 53(3–4), 241–248 (2005). doi:10.1007/s10847-005-0245-6
Szente, L., Fenyvesi, E.: Cyclodextrin-lipid complexes: cavity size matters. Struct. Chem. 28(2), 479–492 (2017)
Sortino, S., Guiffrida, S., De Guldi, G.: The photochemistry of flutamide and its inclusion complex with beta-cyclodextrin: dramatic effect of microenvironment on the nature and on the efficiency of the photodegradation pathways. Photochem. Photobiol. 73, 6–13 (2001)
Yonezawa, Y., Maruyama, S., Takagi, K.: Stability of inclusion complexes of cyclodextrins with Guaiazulene. Agri. Biol. Chem. 45(2), 505–506 (1981)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97(5), 1325–1358 (1997). doi:10.1021/cr960371r
Thakral, S., Madan, A.K..: Reduction in moisture sensitivity/ uptake of moisture sensitive drugs through adduction in urea. J. Pharm. Innov. 3(4), 249–257 (2008). doi:10.1007/s12247-008-9045-z
Thakral, S., Madan, A.K.: Urea co-inclusion compounds of 13 cis-retinoic acid for simultaneous improvement of dissolution profile, photostability and safe handling characteristics. J. Pharm. Pharmacol. 60(7), 823–832 (2008)
Budhwaar, V., Nanda, A.: Simultaneous improvement of dissolution rate and stability of ramipril by formation of urea inclusion complexes. Int. J. App. Pharm. 5(3), 19–25 (2013)
Mosher, G.: Complexation: cyclodextrins. In: Swarbrick, J. (ed.) Encyclopedia of Pharmaceutical Technology, vol. 2, pp. 671–696. Informa Health Care, New York (2007)
Stella, V.J., He, Q.: Cyclodextrins. Toxicol. Pathol. 36(1), 30–42 (2008)
Reeder, R.F., Harbaugh, R.E.: Administration of intravenous urea and normal saline for the treatment of hyponatremia in neurosurgical patients. J. Neurosurg. 70(2), 201–206 (1989)
Nelson, D.L., Cox, M.M. (eds.): Lehinger Principles of Biochemistry, 4th edn., pp. 656–688. WH Freeman and Company, New York (2005)
Clanton, D.C.: Non-protein nitrogen in range supplements. J. Anim. Sci. 47, 765–779 (1978)
Woods, B.C.: Effect of inclusion of urea and supplement frequency on intake, digestion and performance of cattle consuming low quality, tallgrassprairie forage. MS Thesis, Manhattan: Kansas State University, (1997)
Dhall, M., Madan, A.K.: Conversion of viscous liquid malathion into free flowing solids through co-inclusion in urea for multiple benefits. J. Incl. Phenom. Macrocycl. Chem. 86(1–2), 135–151 (2016). doi:10.1007/s10847-016-0648-6
Flaherty, R.J., Nshime, B., Delamarre, M., Dejong, S., Scott, P., Lantz, A.W.: Cyclodextrins as complexation and extraction agents for pesticides from contaminated soil. Chemosphere. 91(7), 912–920 (2013). doi:10.1016/j.chemosphere.2013.02.005
Dodziuk, H., Hashimoto, H., Morillo, E., Bilewicz, R., Chmursky, K.: Applications other than pharmaceutical industry. In: H. Dodziuk (eds.) Cyclodextrins and their Complexes: Chemistry, Analytical Methods, Applications, pp. 459–465. Wiley, Weinheim (2006)
Kanani-Al, T., Mackenzie, A.F., Barhakur, N.N.: Soil water and ammonia volatilization relationships with surface-applied nitrogen fertilizer solutions. Soil Sci. Soc. Am. J. 55, 1761–1766 (1991)
Marsh, K.L., Sims, G.K., Mulvaney, R.L.: Availability of urea to autotrophic ammonia-oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil. Biol. Fert. Soil. 42, 137–145 (2005)
Zhou, J., Ritter, H.: Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 1, 1552–1559 (2010). doi:10.1039/C0PY00219D
Hollingsworth, M.D., Zwanziger, U.W., Brown, M.E., Chaney, J.D., Huffman, J.C., Harris, K.D.M., Smart, S.P.: Spring-loading at the molecular level: relaxation of guest-induced strain in channel inclusion compounds. J. Am. Chem. Soc. 121(41), 9732–9733 (1999). doi:10.1021/ja9919534
Shahgaldian, P., Pieles, U.: Cyclodextrin derivatives as chiral supramolecular receptors for enantioselective sensing. Sensors (Basel) 6(6), 593–615 (2006)
Harris, K.D.M.: Aperiodicity in organic materials. In: Harris, K.D.M., Edwards, P.P. (eds.) Turning point in Solid State Materials and Surface Science, Chapter 19, pp. 315–317. .RSC Publishing, Great Britain (2008)
Schlenk, W.: Asymmetric urea inclusion lattice. III Unstable configurational lattice coordination of guest molecules. Justus Liebigs Ann. Chem. 7, 1179–1194 (1973)
Fernandes, C.M., Ramos, P., Falcao, A.C., Veiga, F.J.: Hydrophilic and hydrophobic cyclodextrins in a new sustained release oral formulation of nicardipine: in vitro evaluation and bioavailability studies in rabbits. J. Control. Release. 88(1), 127–134 (2003)
Sinha, V.R., Nanda, A., Kumaria, R.: Cyclodextrins as sustained-release carriers. Pharm. Technol. 44, 36–46 (2002)
Costa, M.M.E., Cabral-Albuquerque, E.C.M., Alves, T.L.M., Pinto, J.C., Fialho, R.L.: Use of polyhydroxybutyrate and ethyl cellulose for coating of urea granules. Agric. Food Chem. 61(42), 9984–9991 (2013). doi:10.1021/jf401185y
Otey, F.H., Trimnell, D., Westhoff, R.P., Shasha, B.S.: Starch matrix for controlled release of urea fertilizer. J. Agric. Food Chem. 32(5), 1095–1098 (1984). doi:10.1021/jf00125a041
Rouelle, H.: Observations on human urine and on that of the cow and horse, compared to each other. J. de Medecine, de Chirurgie et de Pharmacie. 40, 451–468 (1773)
Wohler, F.: Ueber einige Verbindungen aus der Chinonreihe (About some compounds from quinine series). Justus Liebigs Ann. Chem. 69(3), 294–300 (1849)
Brusilow, S.W., Horwich, A.L.: Urea cycle enzymes. In: Scriver, C.R., Beaudet, A.C., Sly, W.S., Childs, B., Kinzler, K., Vogelstein, B. (eds.) The Metabolic Bases of Inherited Disease, 8th edn, pp. 1900–1963. McGraw-Hill Companies Inc., New York (2001)
INCHEM, International Chemical Safety Cards. ICSC: 0595. Urea. OECD Screening Information dataset. By International Program on Chemical safety (1997)
FAO/WHO. Evaluation of certain food additives and contaminants. Thirty-third report of the joint FAO/WHO expert committee on food additives (JECFA). World Health Organ Tech. Rep. Ser. No. 776. (1989)
Sekiguchi, K., Noboru, O.: Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 9(11), 866–872 (1961)
Habib, F.S., Attia, M.A.: Effect of particle size on the dissolution rate of monophenylbutazone solid dispersion in presence of certain additives. Drug Dev. Ind. Pharm. 11(11), 2009–2019 (1985)
Modi, A., Tayade, P.: Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS Pharm. Sci. Tech. 7(3), (2006). doi:10.1208/pt070368
Okonogi, S., Yonemochi, E., Oguchi, T., Puttipipatkhachorn, S., Samamoto, K.: Enhanced dissolution of ursodeoxycholic acid from the solid dispersion. Drug Dev. Ind. Pharm. 23, 1115–1121 (1997)
Zavodnik, V., Stash, A., Tsirelson, V., Vries, R., Feil, D.: Electron density study of urea using TDS-corrected X-ray diffraction data: quantitative comparison of experimental and theoretical results. Acta Cryst B. 55, 45–54 (1999)
Smith, A.E.: The crystal structure of urea-hydrocarbon complexes. Acta Crystallogr. 5, 224–235 (1952)
Thakral, S., Madan, A.K.: Topological models for prediction of adductability of branched aliphatic compounds in urea. J. Incl. Phenom. Macrocycl. Chem. 56, 405–412 (2006). doi:10.1007/s10847-006-9123-0
Thakral, S., Madan, A.K.: Topological models for prediction of adductability of substituted cyclic organic compounds in urea. J. Incl. Phenom. Macrocycl. Chem. 58(3), 321–326 (2007). doi:10.1007/s10847-006-9160-8
Fetterly, L.C.: Organic adducts. In: Mandelcorn, L. (ed.) Non-Stoichiometric Compounds, pp. 491–567. Academic Press, New York (1964)
Swern, D.: Urea and thiourea complexes in separating organic compounds. Ind. Eng. Chem. 47, 216–221 (1955)
McAdie, H.D.: Thermal decomposition of molecular complexes. III Urea inclusion compounds of monosubstituted aliphatic series. Can. J. Chem. 41, 2144–2153 (1963)
Zimmerschied, W.J., Dinerstein, R.A., Wietkamp, A.W., Marschner, R.F.: Crystalline adducts of urea with linear aliphatic compounds. Ind. Eng. Chem. 42, 1300–1306 (1950)
Thakral, S., Madan, A.K.: Urea inclusion compounds of enalapril maleate for the improvement of pharmaceutical characteristics. J. Pharm. Pharmacol. 59(11), 1501–1507 (2007)
Thakral, S., Madan, A.K.: Urea co-inclusion compounds of glipizide for the improvement of dissolution profile. J. Incl. Phenom. Macrocycl. Chem. 60(3), 203–209 (2008). doi:10.1007/s10847-007-9368-2
Thakral, S., Madan, A.K.: Adduction of amiloride hydrochloride in urea through a modified technique for the dissolution enhancement. J. Pharm. Sci. 97(3), 1191–1201 (2008)
Dhall, M., Madan, A.K.: Studies on urea co-inclusion complexes of simvastatin for improvement of pharmaceutical characteristics. J. Incl. Phenom. Macrocycl. Chem. 81(1–2), 105–120 (2015). doi:10.1007/s10847-014-0439-x
Dhall, M., Madan, A.K..: Simultaneous improvement in dissolution profile and content uniformity of lafutidine through co-inclusion in urea. J. Incl. Phenom. Macrocycl. Chem. 82(3–4), 335–350 (2015). doi:10.1007/s10847-015-0493-z
Dhall, M., Madan, A.K.: Steep improvement in dissolution profile of ezetimbe through co-inclusion in urea. J. Pharm. Invest. 46(1), 1–19 (2016). doi:10.1007/s40005-016-0236-1
Vinod Budhwaar V., Nanda, A.: Preparation and evaluation of urea co-inclusion complexes of Co-Q10 for the simultaneous enhancement of dissolution profile and its stability. Int. J. Chem.Pharm. Sci. 3(6), 1787–1794 (2015)
Thakral, S., Madan, A.K..: Topological models for prediction of heat of decomposition of urea inclusion compounds containing aliphatic endocytes. J. Incl. Phenom. Macrocycl. Chem. 60(1), 187–192 (2008). doi:10.1007/s10847-007-9345-9
Thakral, S., Madan, A.K..: Topological models for the prediction of host: guest ratio of urea inclusion compounds. J. Incl. Phenom. Macro. Chem. 65(3–4), 411–417 (2009). doi:10.1007/s10847-009-9583-0
Dhall, M., Madan, A.K.: Thermal and other analytical studies on bifenthrin urea co-inclusion complex—A human guarded insecticide formulation. J. Therm. Anal. Calorim. doi:10.1007/s10973-016-6072-8 (2017)
Dhall, M., Madan, A.K.: Preparation, characterization and evaluation of human guarded chlorpyrifos urea co-inclusion complexes. Indian J. Pharm. Sci. 79(1), 91–104 (2017)
Dhall, M., Madan, A.K.: Urea complexes of chlorpyrifos, malathion, bifenthrin and cypermethrin for improving safe handling and other characteristics. Indian Patent No. 201611002986 filed on 28 Jan (2016)
Bajaj, V., Madan, A.K.: Highly distorted urea based channel complexes as an alternative to solid dispersions for improving content uniformity and dissolution rate. Proceedings of the First Regional Conference of IEEE Engineering in Medicine & Biology Society and 14th Conference of the Biomedical Engineering Society of India—An International Meet, New Delhi, 4.67–4.68 (1995)
Thakral, S., Madan, A.K.: Improvement of dissolution profile of gliclazide through co-inclusion in urea. British Pharmaceutical Conference, Manchester, UK, 7–9th Sept (2008)
Dhall, M., Madan, A.K.: Studies on urea co-inclusion complexes of ebastine for steep improvement in dissolution profile. Indian Drugs. 54(8), 42–53 (2017) (In-press)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhall, M., Madan, A.K. Comparison of cyclodextrins and urea as hosts for inclusion of drugs. J Incl Phenom Macrocycl Chem 89, 207–227 (2017). https://doi.org/10.1007/s10847-017-0748-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0748-y