Abstract
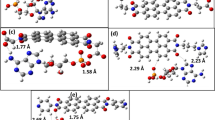
[M05-2X/6-31G*:PM3MM] and [B3LYP/6-31G*:PM3] ONIOM2 methods have been used to investigate the vitamin A propionate/β cyclodextrin complex with 1:2 stoichiometry. Both methods give almost the same lowest energy minimum. The minimum energy structure of the complex is found in good agreement with experimental data. In this configuration, the major structure of propionate of vitamin A (PVA) is embedded inside the two cavities of βCD while the propionate group is kept outside. However, the three methyl groups of PVA are positioned in the free space between both βCD molecules. The driving forces for complexation are dominated by Van der Waals interactions between PVA and the βCD molecules assisted with multiple hydrogen bond interactions between the two cyclodextrin molecules. These interactions were investigated using the natural bond orbital approach.








Similar content being viewed by others
References
Loveday, S.M., Singh, H.: Trends in food science & technology 19, 657–668 (2008)
Weisse, S.: Doctorat thesis serie N° 741 Faculté de Pharmacie de Chatenay-Malabry Université de Paris XI (2002)
Villiers, A.: Sur la fermentation de la fécule par l’action du ferment butyriqué. C.R. Hebd. Seances Acad. Sci. 112, 536–538 (1891)
Frömming, K.H., Szejtli, J.: Cyclodextrins in Pharmacy. Kluwer Academic Publishers, Dordrecht (1994)
Attoui Yahia, O., Khatmi, D.E.: J Mol. Struct. (Theochem) 912, 38–43 (2009)
Loftsson, T., Jarho, P., Másson, M., Järvinen, T.: Expert Opin Drug. Deliv. 2, 335–351 (2005)
Huang, M.J., Quan, Z., Liu, Y.M: Int. J. Quantum Chem. 109, 81–90 (2009)
Palmieri, F., Wehrlé, P., Duportail, G., Stamm, A.: Drug Dev. Ind. Pharm. 18(19), 2117 (1992)
Muñoz-Botella, S., Martín, M.A., Del Castillo, B., Lerner, D.A., Menéndez, J.C.: Analytica. Chimica. Acta. 468, 161–170 (2002)
Stewart, J.J.P.: MOPAC2009, Stewart Computational Chemistry. Colorado Springs, CO, USA. http://OpenMOPAC.net (2008)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A.Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C.,Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari K, Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., M. Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision E.01. Gaussian Inc., Pittsburgh PA (2009)
Barbiric, D.J., de Rossi, R.H., Castro, E.A.: J. Mol. Struct. (Theochem) 537, 234–235 (2001)
Matei, I., Nicolae, A., Hillebrand, M.: J. Incl. Phenom. Macrocycl. Chem. 57, 597–601 (2007)
Yan, C., Xiu, Z., Li, X., Teng, H., Hao, C.: J. Incl. Phenom. Macrocycl. Chem. 58, 337 (2007)
Dapprich, S., Koma′romi, I., Byun, K.S., Morokuma, K., Frisch, M.J.: J. Mol. Struct. (Theochem) 461–462, 1–21 (1999)
Kuno, M., Hannongbua, S., Morokuma, K.: Chem. Phys. Lett. 380, 456–463 (2003)
James, C., Amal Raj, A., Reghunathan, R., Joe, I.H., Jayakumar, V.S.: J. Raman Spectrosc. 37, 1381 (2006)
Liu, J.N., Chen, Z.R., Yuan, S.F.: J. Zhejiang Univ. Sci. B6, 584 (2005)
Szafran, M., Komasa, A., Adamska, E.B.: J. Mol. Struct. (Theochem) 827, 101 (2007)
Kavitha, E., Sundaraganesan, N., Sebastian, S., Kurt, M.: Spectrochim. Acta Part A 77, 612–619 (2010)
Zhao, Y., Truhlar, D.G.: Org. Lett. 8, 5753 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mallem, N., Khatmi, D., Azzouz, S. et al. Computational studies of 1:2 complex between retinol propionate and β cyclodextrin. J Incl Phenom Macrocycl Chem 73, 305–312 (2012). https://doi.org/10.1007/s10847-011-0057-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-0057-9