Abstract
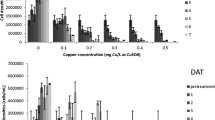
The marine dinoflagellate Cochlodinium polykrikoides has spread worldwide and is responsible for harmful algal blooms. The chemical biocides, copper sulfate (CuSO4) and sodium hypochlorite (NaOCl), are known to be effective in removing bloom-forming or biofouling organisms. Here, we assessed the biocidal efficiency and toxicological properties of NaOCl and CuSO4 on the physiological and catalase responses of C. polykrikoides. The endpoints used were cell counts, pigment content, chlorophyll autofluorescence (CAF), and antioxidant catalase (CAT) activity. The test organism showed a dose-dependent decrease in growth rate against the algicides; 72-h median effective concentrations (EC50) were 0.584 and 0.633 mg L–1 for NaOCl and CuSO4, respectively. The decrease in pigment levels and CAF intensity showed that NaOCl and CuSO4 might affect the photosynthetic processes of the exposed cells. Furthermore, a considerable increase in CAT activity in the cells was detected, indicating that the algicides might generate reactive oxygen species, thereby markedly damaging the cells. These results suggest that the test algicides are very effective in removing C. polykrikoides by inducing cellular stress and inhibiting cell recovery at higher concentrations.






Similar content being viewed by others
References
Abdul-Baki AA (1974) Pitfalls in using sodium hypochlorite as a seed disinfectant in 14C incorporation studies. Plant Physiol 53:768–771
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Albrich JM, Mccarthy CA, Hurst JK (1981) Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A 78:210–214
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Anderson DM (2007) The ecology and oceanography of harmful algal blooms: multidisciplinary approaches to research and management. IOC Technical Series 74, UNESCO 2007, IOC/2007/TS/74. http://lib.ruppin.ac.il/multimedia_michmoret/Anderson_BruunMemorialLecture2005.pdf. Accessed 29 Jan 2014
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Bonini MG, Siraki AG, Bhattacharjee S, Mason RP (2007) Glutathione-induced radical formation on lactoperoxidase does not correlate with the enzyme's peroxidase activity. Free Radic Biol Med 42:985–992
Calderon RL (2000) The epidemiology of chemical contaminants of drinking water. Food Chem Toxicol 38:513–520
Cervantes C, Gutierrez-Corona F (1994) Copper resistance mechanisms in bacteria and fungi. FEMS Microbiol Rev 14:121–137
Ebenezer V, Ki J-S (2013) Physiological and biochemical responses of the marine dinoflagellate Prorocentrum minimum exposed to the oxidizing biocide chlorine. Ecotoxicol Environ Saf 92:129–134
Gant VA, Wren MWD, Rollins MSM, Jeanes A, Hickok SS, Hall TJ (2007) Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms. J Antimicrob Chemother 62:294–299
García-Villada L, Rico M, Altamirano M, Sánchez-Martín L, Lopez-Rodas V, Costas E (2004) Occurrence of copper resistant mutants in the toxic cyanobacterium Microcystis aeruginosa: characterization and future implications in the use of copper sulphate as an algaecide. Water Res 38:2207–2213
Gómez JM, Jiménez A, Olmos E, Sevilla F (2004) Location and effects of long-term NaCl stress on superoxide dismutase and ascorbate peroxidase isoenzymes of pea (Pisum sativum cv. Puget) chloroplasts. J Exp Bot 55:119–130
Gregg MD, Hallegraef GM (2007) Efficacy of three commercially available ballast water biocides against vegetative microalgae, dinoflagellate cysts and bacteria. Harmful Algae 6:567–584
Guillard RRL, Ryther JH (1962) Studies of marine plankton diatoms. I. Cyclotella nana Hustedt and Detonula covervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guo R, Ki JS (2012) Differential transcription of heat shock protein 90 (HSP90) in the dinoflagellate Prorocentrum minimum by copper and endocrine-disrupting chemicals. Ecotoxicology 21:1448–1457
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Han FX, Hargreaves JA, Kingery WL, Huggett DB, Schlenk DK (2001) Accumulation, distribution, and toxicity of copper in sediments of catfish ponds receiving periodic copper sulfate applications. J Environ Qual 30:912–919
Haas CN (1999) Disinfection. In: Letterman RD (ed) Water quality and treatment a handbook of community water supplies. American Water Works Association 5th edn. McGraw-Hill, New York, pp 14.1–14.60.
Hilgren J, Swanson KM, Diez-Gonzalez F, Cords B (2007) Inactivation of Bacillus anthracis spores by liquid biocides in the presence of food residue. Appl Environ Microbiol 73:6370–7
Hinton DE, Lauren DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes potential bio-markers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 17–57
Jegatheesan V, Kim S-H, Joo CK, Baoyu G (2009) Evaluating the effects of granular and membrane filtrations on chlorine demand in drinking water. J Environ Sci 21:23–29
Jeong HJ, Kim HR, Kim KI, Kim KY, Park KH, Kim ST, Yoo YD, Song JY, Kim JS, Seong KA, Yih WH, Pae SJ, Lee CH, Huh MD, Lee SH (2002) NaOCl produced by electrolysis of natural seawater as a potential method to control marine red tide dinoflagellates. Phycologia 41:643–656
Kim CK, Lee SG, Kim HG (2000) Biochemical responses of fish exposed to a harmful dinoflagellate Cochlodinium polykrikoides. J Exp Mar Biol Ecol 254:131–141
Kim DI, Matsuyama Y, Nagasoe S, Yamaguchi M, Yoon YH, Oshima Y, Imada N, Honjo T (2004) Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J Plankton Res 26:61–66
Kim HG (2006) Mitigation and controls of HABs. Ecological Studies 189:327–338
Kim J-G, Kim B, Lee C-G (2007) Alga-lytic activity of Pseudomonas fluorescens against the red tide causing marine alga Heterosigma akashiwo (Raphidophyceae). Biol Control 41:296–303
Kudela RM, Gobler CJ (2012) Harmful dinoflagellate blooms caused by Cochlodinium sp.: global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14:71–86
Lee YS (2008) Utilization of various nitrogen, phosphorus, and selenium compounds by Cochlodinium polykrikoides. J Environ Biol 29:799–804
Letelier ME, Lepe AM, Faundez M, Salazar J, Marin R, Aracena P, Speisky H (2005) Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact 151:71–82
Li Y, Trush MA (1993) DNA damage resulting from the oxidation of hydroquinone by copper: role for a Cu(II)/Cu(I)) redox cycle and reactive oxygen generation. Carcinogenesis 14:1303–1311
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Ma Z, Gao K, Li W, Xu Z, Lin H, Zheng Y (2011) Impacts of chlorination and heat shocks on growth, pigments and photosynthesis of Phaeodactylum tricornutum (Bacillariophyceae). J Exp Mar Biol Ecol 397:214–219
Mahawar M, Tran V, Sharp JS, Maier RJ (2011) Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase. J Biol Chem 286:19159–19169
Nancharaiah YV, Rajadurai M, Venugopalan VP (2007) Single cell level microalgal ecotoxicity assessment by confocal laser scanning microscopy and digital image analysis. Environ Sci Technol 41:2617–2621
Ogawa K (2005) Glutathione - associated regulation of plant growth and stress responses. Antioxid Redox Signal 7:973–981
Oliveira-Filho ECD, Lopes RM, Paumgartten FJER (2004) Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 56:369–374
Organisation for Economic Cooperation and Development (OECD) (2011) OECD guidelines for the testing of chemicals, Test no. 201: freshwater algal and cyanobacteria growth inhibition test. OECD Publications, Paris
Oukarroum A, Perreault F, Popovic R (2012) Interactive effects of temperature and copper on photosystem II photochemistry in Chlorella vulgaris. J Photochem Photobiol B 110:9–14
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, p 84
Patil JS, Jagadeesan V (2011) Effect of chlorination on the development of marine biofilms dominated by diatoms. Biofouling 27:241–254
Phe MH, Dossot M, Guilloteau H, Block JC (2005) Nucleic acid fluorochromes and flow cytometry prove useful in assessing the effect of chlorination on drinking water bacteria. Water Res 39:3618–3628
Qian H, Yu S, Sun Z, Xie X, Liu W, Fu Z (2010) Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat Toxicol 99:405–12
Radocanović TB, Borković-Mitić SS, Perendija BR, Despotović SG, Pavlović SZ, Cakić PD, Saičić ZS (2010) Superoxide dismutase and catalase activities in the liver and muscle of barbel (Barbus barbus) and its intestinal parasite (Pomphoryinchus laevis) from the Danube river, Serbia. Arch Biol Sci 62:97–105
Raman RK, Cook BC (1990) Guidelines for applying copper sulphate as an algicide: Lake Laomi field study, vol 217. Illinois State Water Survey, Springfield, pp 785–2800
Richlen MA, Morton SL, Jamali EA, Rajan A, Anderson DA (2010) The catastrophic 2008-2009 red tide in the Arabian Gulf region, with observations on the identification and phylogeny of fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9:163–172
Santo CE, Taudte N, Nies DH, Grass G (2008) Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl Environ Microbiol 74:977–986
Sengo MR, Anderson DM (2004) Controlling harmful algal blooms through clay flocculation. J Eukaryot Microbiol 51:169–172
Shao H-B, Chu L-Y, Lu Z-H, Kang C-M (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int J Biol Sci 4:8–14
Song Y-C, Sivakumar S, Woo J-H, Ko S-J, Hwang E-J, Jo Q (2010) Removal of Cochlodinium polykrikoides by dredged sediment: a field study. Harmful Algae 9:227–232
Soto P, Gaete H, Hidalgo ME (2011) Assessment of catalase activity, lipid peroxidation, chlorophyll-a and growth rate in the freshwater green algae Pseudokirchneriella subcapitata exposed to copper and zinc. Lat Am J Aquat Res 39:280–285
Stauber JL, Franklin NM, Adams MS (2002) Applications of flow cytometry to ecotoxicity testing using microalgae. Trends Biotechnol 20:141–143
Stiborová M, Doubravová M, Březinova A, Friedrich A (1986) Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of barley (Hordeum vulgare L.). Photosynthetica 20:418–425
Teisseyre A, Mozrzymas JW (2006) The inhibitory effect of copper ions on lymphocyte KVI.3 potassium channels. J Physiol Pharmacol 57:301–314
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Trampe E, Kolbowski J, Schreiber U, Kühl M (2011) Rapid assessment of different oxygenic phototrophs and single-cell photosynthesis with multicolour variable chlorophyll fluorescence imaging. Mar Biol 158:1667–1675
Trivedi MH, Sangai NP, Renuka A (2012) Assessment of toxicity of copper sulphate pentahydrate on oxidative stress indicators on liver of gold fish (Carassius auratus). Bull Environ Pharmacol Life Sci 1:52–57
U.S. Environmental Protection Agency (USEPA) (2009) Reregistration eligibility decision (RED) for coppers. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/oppsrrd1/REDs/copper_red_amend.pdf. Accessed 29 Jan 2014
Verhoeven RL, Eloff JN (1979) Effect of lethal concentrations of copper on the ultrastructure and growth of Microcystis. Proc Electron Microsc Soc South Afr 9:161–162
Viriyatum R (2013) Effectiveness of coated, controlled-release copper sulfate as an algicide for phytoplankton control in ponds. PhD dissertation, Auburn University, Auburn, Alabama. http://hdl.handle.net/10415/3585. Accessed 29 Jan 2014
Watson CA, Yanong RPE (2006) Use of copper in freshwater aquaculture and farm ponds. University of Florida, Gainesville. FL. http://edis.ifas.ufl.edu/FA008. Accessed 29 Jan 2014
White GC (1999) Handbook of chlorination and alternative disinfectants, 4th edn. John Wiley and Sons Inc., New York, pp 212–287
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (nos. 2012-0001741, 2013-044476), a grant from the National Fisheries Research and Development (NFRDI) funded to J.-S. Ki, and a 2014 Research Grant from Sangmyung University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
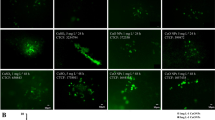
Variation in chlorophyll autoflourescence in C. polykrikoides after 6 and 72 h exposure to algicides NaOCl (upper panel) and CuSO4 (lower panel). Scale = 20 μm. (PPT 491 kb)
Rights and permissions
About this article
Cite this article
Ebenezer, V., Lim, W.A. & Ki, JS. Effects of the algicides CuSO4 and NaOCl on various physiological parameters in the harmful dinoflagellate Cochlodinium polykrikoides . J Appl Phycol 26, 2357–2365 (2014). https://doi.org/10.1007/s10811-014-0267-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0267-9