Abstract
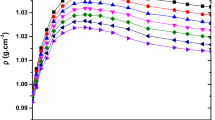
Densities, viscosities, and refractive indices of three binary systems consisting of 1-butanol with polyethylene glycols of different molecular weights (PEG 200 and PEG 400) or tetraethylene glycol dimethyl ether (TEGDME) were measured at ten temperatures (288.15, 293.15, 298.15, 303.15, 308.15, 313.15, 318.15, 323.15, 328.15, and 333.15) K and atmospheric pressure. Densities of the selected binary mixtures were measured with an Anton Paar DMA 5000 digital vibrating U-tube densimeter, refractive indices were measured with an automatic Anton Paar RXA-156 refractometer, while for viscosity measurements, a digital Stabinger SVM 3000/G2 viscometer was used. From these data, excess molar volumes were calculated and fitted to the Redlich–Kister equation. The obtained results have been analyzed in terms of specific molecular interactions and mixing behavior between mixture components, as well as the influence of temperature on them. Viscosity data were also correlated by Grunberg–Nissan, Eyring–UNIQUAC, three-body McAlister, and Eyring–NRTL models.

Similar content being viewed by others
References
J. Liang, Lv. Jing, J. Fan, Z. Shang, Synth. Commun. 39, 2822 (2009)
I.-W. Kim, M.D. Jang, Y.K. Ryu, E.H. Cho, Y.K. Lee, J.H. Park, Anal. Sci. 18, 1357 (2002)
Z.P. Višak, L.M. Ilharco, A.R. Garcia, V. Najdanović-Višak, J.M.N.A. Farleira, F.J.P. Caetano, M.L. Kijevčanin, S.P. Šerbanović, J. Phys. Chem. B 115, 8481 (2011)
O.E. Philippova, S.I. Kuchanov, I.N. Topchieva, V.A. Kabanov, Macromolecules 18, 1628 (1985)
Toxicological Evaluation of Certain Food Additives, Food Additives Series 14 (World Health Organization, Geneva, 1979)
Code of Federal Regulations, Title 21, vol. 3 (FDA, Washington, DC, 2001)
J.M. Harris, Poly(Ethylene Glycol) Chemistry: Bio-technical and Bio-medical Applications (Plenum Press Inc., New York, 1992), pp. 7–12
M.E. Ferreyra de Ruiz Holgado, C.R. de Schaefer, E.L. Arancibia, J. Chem. Eng. Data 47, 144 (2002)
M.A. Monsalvo, A. Baylaucq, P. Reghem, S.E. Quiñones-Cisneros, C. Boned, Fluid Phase Equilib. 233, 1 (2005)
F. Han, J. Zhang, G. Chen, X. Wei, J. Chem. Eng. Data 53, 2598 (2008)
C.N. Schubert, W.I. Echter, The Method of Polymer Ethylene Glycol for Removal Pollution from Gases, CN. Patent 1364096A (2002)
M. Heisel, A. Belloni, Gas. Sep. Purif. 5, 111 (1991)
O. Redlich, A. Kister, Ind. Eng. Chem. 40, 345 (1948)
L. Grunberg, A.H. Nissan, Nature 164, 799 (1949)
R.J. Martins, M.J.E.D. Cardoso, O.E. Barcia, Ind. Eng. Chem. Res. 39, 849 (2000)
R.A. McAllister, AIChE J. 6, 427 (1960)
L.T. Novak, Ind. Eng. Chem. Res. 43, 2602 (2004)
A.Ž. Tasić, D.K. Grozdanić, B.D. Djordjević, S.P. Šerbanović, N. Radojković, J. Chem. Eng. Data 40, 586 (1995)
A.R. Riddick, W.B. Bunger, T.K. Sakano, Organic Solvents, Physical Properties and Methods of Purification, 4th edn. (Wiley, New York, 1986)
Z. Shan, A.F.A. Asfour, J. Chem. Eng. Data 44, 118 (1999)
TRC Thermodynamic Tables, Hydrocarbons, Thermodynamic Research Center (The Texas A &M University System, College Station, TX, 1998)
S. Ottani, D. Vitalini, F. Comelli, C. Castellari, J. Chem. Eng. Data 47, 1197 (2002)
E.A. Mueller, P. Rasmussen, J. Chem. Eng. Data 36, 214 (1991)
C. Aucouturier, G. Roux-Desgrenges, A.H. Roux, J. Chem. Thermodyn. 31, 289 (1999)
A. Pal, G. Dass, A. Kumar, J. Chem. Eng. Data 44, 2 (1999)
J.N. Real, T.P. Iglesias, S.M. Pereira, M.A. Rivas, J. Chem. Thermodyn. 34, 1029 (2002)
F.J. Carmona, F.J. Arroyo, I.D. de la Fuente, J.A. Gonzales, J.C. Cobos, Can. J. Chem. 77, 1608 (1999)
D. Bajić, G. Ivaniš, Z. Višak, E. Živković, S. Šerbanović, M. Kijevčanin, J. Chem. Thermodyn. 57, 510 (2013)
D.W. Marquardt, J. Soc. Ind. Appl. Math. 2, 431 (1963)
Acknowledgments
The authors gratefully acknowledge the financial support received from the Research Fund of Ministry of Science and Environmental Protection, Serbia and the Faculty of Technology and Metallurgy, University of Belgrade (Project No. 172063).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Živković, N., Šerbanović, S., Kijevčanin, M. et al. Volumetric Properties, Viscosities, and Refractive Indices of the Binary Systems 1-Butanol + PEG 200, + PEG 400, and + TEGDME. Int J Thermophys 34, 1002–1020 (2013). https://doi.org/10.1007/s10765-013-1469-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-013-1469-0