Abstract
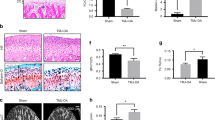
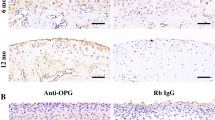
Although the up-regulation of periostin in osteoarthritic (OA) is found, its function on OA condyle caused by disc displacement is not clear. Our objective was to explore whether periostin has any effect on condylar resorption. We initially identified periostin-positive cells in temporomandibular joint osteoarthritic (TMJ-OA) cartilage. Furthermore, the vitro analysis confirmed that the expression of periostin in chondrocytes treated with a static pressure of 150 kpa and 200 kpa for 3 h by an in-house-designed pressure chamber. To explore the underlying mechanism, we found that periostin can induce IκBα phosphorylation and its subsequent degradation, leading to consequent p65 nuclear translocation and subsequent induction of ADAMTS5 expression, which is known to be detrimental to cartilage extracellular matrix production. Importantly, inhibiting NF-κB signaling, by BAY 11-7082 treatment, rescued periostin-induced ADAMTS5 up-regulation. This study elucidated the direct role of periostin in condylar resorption, which was found to occur via NF-κB-ADAMTS5 signaling. Inhibition of this pathway might provide a new strategy for TMJ-OA treatment.





Similar content being viewed by others
References
Meyers, R.A., K.P. Schellhas, H.D. Hall, A.T. Indresano, and C.H. Wilkes. 1992. Guidelines for diagnosis and management of disorders involving the temporomandibular joint and related musculoskeletal structures. American Society of Temporomandibular Joint Surgeons. Northwest Dentistry 71: 21–27.
Wilkes, C.H. 1990. Internal derangements of the temporomandibular joint. Pathological variations. Northwest Dentistry 69: 25–32.
Chijimatsu, R., Y. Kunugiza, Y. Taniyama, N. Nakamura, T. Tomita, and H. Yoshikawa. 2015. Expression and pathological effects of periostin in human osteoarthritis cartilage. BMC Musculoskeletal Disorders 16: 215.
Chou, C.H., C.C. Wu, I.W. Song, H.P. Chuang, L.S. Lu, J.H. Chang, S.Y. Kuo, C.H. Lee, J.Y. Wu, Y.T. Chen, V.B. Kraus, and M.T. Lee. 2013. Genome-wide expression profiles of subchondral bone in osteoarthritis. Arthritis Research & Therapy 15: R190.
Bonnet, N., P. Garnero, and S. Ferrari. 2016. Periostin action in bone. Molecular and Cellular Endocrinology 432: 75–82.
Copray, J.C., H.W. Jansen, and H.S. Duterloo. 1985. An in -vitro system for studying the effect of variable compressive forces on the mandibular condylar cartilage of the rat. Archives of Oral Biology 30: 305–311.
Mrosek, E.H., A. Lahm, C. Erggelet, M. Uhl, H. Kurz, B. Eissner, and J.C. Schagemann. 2006. Subchondral bone trauma causes cartilage matrix degeneration: an immunohistochemical analysis in a canine mode. Osteoarthritis and Cartilage 14: 171–178.
Roh, H.S., W. Kim, Y.K. Kim, and J.Y. Lee. 2012. Relationships between disk displacement, joint effusion, and degenerative changes of the TMJ in TMD patients based on MRI findings. Journal of Cranio-Maxillo-Facial Surgery 40: 283–286.
Zhang, S., D. Huang, X. Liu, C. Yang, G. Undt, S.M. Haddad, and Z. Chen. 2011. Arthroscopic treatment for intraarticular adhesions of the temporomandibular joint. Journal of Oral and Maxillofacial Surgery 69: 2120–2127.
Kudo, A. 2011. Periostin in fibrillogenesis for tissue re-generation: periostin actions inside and outside the cell. Cellular and Molecular Life Sciences 68: 3201–3207.
Litvin, J., A.H. Selim, M.O. Montgomery, K. Lehmann, M.C. Rico, H. Devlin, D.P. Bednarik, and F.F. Safadi. 2004. Expression and function of periostin-isoforms in bone. Journal of Cellular Biochemistry 92: 1044–1061.
Kubomura, D., T. Ueno, M. Yamada, and I. Nagaoka. 2017. Evaluation of the chondroprotective action of N-acetylglucosamine in a rat experimental osteoarthritis model. Experimental and Therapeutic Medicine 14: 3137.
Tajika, Y., T. Moue, S. Ishikawa, K. Asano, T. Okumo, H. Takagi, and T. Hisamitsu. 2017. Influence of periostin on synoviocytes in knee osteoarthritis. Vivo 31: 69.
Loeser, R.F., A.L. Olex, M.A. McNulty, C.S. Carlson, M.F. Callahan, C.M. Ferguson, J. Chou, X. Leng, and J.S. Fetrow. 2012. Microarray analysis reveals age-related differences in gene expression during the development of osteoarthritis in mice. Arthritis and Rheumatism 64: 705–717.
Gerbaix, M., L. Vico, S.L. Ferrari, and N. Bonnet. 2015. Periostin expression contributes to cortical bone loss during unloading. Bone 71: 94–100.
Merle, B., and P. Garnero. 2012. The multiple facets of periostin in bone metabolism. Osteoporosis International 23: 1199–1212.
Rammelsberg, P., P.R. Pospiech, L. Jäger, J.M. Pho Duc, A.O. Böhm, and W. Gernet. 1997. Variability of disk position in asymptomatic volunteers and patients with internal derangements of the TMJ. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 83: 393–399.
Attur, M., Q. Yang, K. Shimada, Y. Tachida, H. Nagase, P. Mignatti, L. Statman, G. Palmer, T. Kirsch, F. Beier, and S.B. Abramson. 2015. Elevated expression of periostin in human osteoarthritic cartilage and its potential role in matrix degradation via matrix metalloproteinase-13. The FASEB Journal 23: 4107–4121.
Scatena, M., and C. Giachelli. 2002. The alpha(v)beta3 integrin, NF-kappaB, osteoprotegerin endothelial cell survival pathway. Potential role in angiogenesis. Trends in Cardiovascular Medicine 12: 83.
Smith, R.L., S.F. Rusk, B.E. Ellison, P. Wessells, K. Tsuchiya, D.R. Carter, W.E. Caler, L.J. Sandell, and D.J. Schurman. 1996. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. Journal of Orthopaedic Research 14: 53–60.
Huang, L., X. Cai, H. Li, Q. Xie, M. Zhang, and C. Yang. 2015. The effects of static pressure on chondrogenic and osteogenic differentiation in condylar chondrocytes from temporomandibular joint. Archives of Oral Biology 60: 622–630.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods 25: 402–408.
Funding
This study was supported by the National Natural Science Foundation of China in 2016 (81671010) and Western Medicine Guidance Project of Shanghai Science and Technology Commission(16411960800)..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
All human tissues were collected according to the informed protocol approved by Independent Ethics Committee of Shanghai Ninth People’s Hospital affiliated to Shanghai JiaoTong University, School of Medicine.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, B., Liu, X., Chen, X. et al. Periostin Mediates Condylar Resorption via the NF-κB-ADAMTS5 Pathway. Inflammation 43, 455–465 (2020). https://doi.org/10.1007/s10753-019-01129-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01129-4