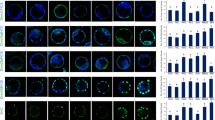
Abstract
Propofol is a widely used intravenous anesthetic. The aim of this study was to investigate the roles of nuclear factor erythroid-2-related factor 2 (Nrf2) and NADPH oxidase (NOX) in propofol protection in inflammatory conditions induced by lipopolysaccharide (LPS). Human alveolar epithelial cells (A549 cell line) were incubated with propofol (10, 25, and 50 μmol/L) for 1 h and then treated with LPS (100 ng/mL) for 24 h. Results indicated that propofol not only attenuated LPS-induced expression of iNOS, NOX, and COX2, but decreased the production of ROS, NO, and PGE2 as well. Propofol also increased the GSH levels and the mRNA and protein levels of Nrf2. Notably, Nrf2 siRNA and the inhibitors of COX-2 and NOX attenuated the inhibition of propofol on ROS production. In conclusion, propofol reduced LPS-induced ROS production via inhibition of inflammatory factors and enhancement of Nrf2-related antioxidant defense, providing its cytoprotective evidence under inflammatory conditions.






Similar content being viewed by others
References
Riedemann, N.C., R.F. Guo, and P.A. Ward. 2003. Novel strategies for the treatment of sepsis. Nature Medicine 9: 517–24.
Brigham, K.L., and B. Meyrick. 1986. Endotoxin and lung injury. The American Review of Respiratory Disease 133: 913–27.
Callahan, L.A., D. Nethery, D. Stofan, A. DiMarco, and G. Supinski. 2001. Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. American Journal of Respiratory Cell and Molecular Biology 24: 210–7.
Lambeth, J.D. 2004. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology 4: 181–9.
Touyz, R.M., A.M. Briones, M. Sedeek, D. Burger, and A.C. Montezano. 2011. NOX isoforms and reactive oxygen species in vascular health. Molecular Interventions 11: 27–35.
Allaouchiche, B., R. Debon, J. Goudable, D. Chassard, and F. Duflo. 2001. Oxidative stress status during exposure to propofol, sevoflurane and desflurane. Anesthesia & Analgesia 93: 981–5.
Chu, C.H., D. David Liu, Y.H. Hsu, K.C. Lee, and H.I. Chen. 2007. Propofol exerts protective effects on the acute lung injury induced by endotoxin in rats. Pulmonary Pharmacology & Therapeutics 20: 503–12.
Hsing, C.H., W. Chou, J.J. Wang, H.W. Chen, and C.H. Yeh. 2011. Propofol increases bone morphogenetic protein-7 and decreases oxidative stress in sepsis-induced acute kidney injury. Nephrology Dialysis Transplantion 26: 1162–72.
Song, X.M., Y.L. Wang, J.G. Li, et al. 2009. Effects of propofol on pro-inflammatory cytokines and nuclear factor kappaB during polymicrobial sepsis in rats. Molecular Biology Reports 36: 2345–51.
Chiu, W.T., Y.L. Lin, C.W. Chou, and R.M. Chen. 2009. Propofol inhibits lipoteichoic acid-induced iNOS gene expression in macrophages possibly through downregulation of Toll-like receptor 2-mediated activation of Raf-MEK1/2-ERK1/2-IKK-NFkappaB. Chemico-Biological Interactions 181: 430–9.
Chuquimia, O.D., D.H. Petursdottir, M.J. Rahman, K. Hartl, and M. Singh. 2012. Fernandez C The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems. PloS One 7: e32125.
Lin, Y., M. Zhang, and P.F. Barnes. 1998. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infection and Immunity 66: 1121–6.
Matthay, M.A., and R.L. Zemans. 2011. The acute respiratory distress syndrome: pathogenesis and treatment. Annual Review of Pathology: Mechanisms of Disease 6: 147–63.
Razavi, H.M., L. Wang, S. Weicker, et al. 2005. Pulmonary oxidant stress in murine sepsis is due to inflammatory cell nitric oxide. Critical Care Medicine 33: 1333–9.
Wang, L., R. Taneja, W. Wang, et al. 2013. Human alveolar epithelial cells attenuate pulmonary microvascular endothelial cell permeability under septic conditions. PloS One 8: e55311.
Kaspar, J.W., S.K. Niture, and A.K. Jaiswal. 2009. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biology & Medicine 47: 1304–9.
Kensler, T.W., N. Wakabayashi, and S. Biswal. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology 47: 89–116.
Cho, H.Y., S.P. Reddy, A. Debiase, M. Yamamoto, and S.R. Kleeberger. 2005. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radical Biology & Medicine 38: 325–43.
Redl, H., S. Bahrami, G. Schlag, and D.L. Traber. 1993. Clinical detection of LPS and animal models of endotoxemia. Immunobiology 187: 330–45.
Shih, Y.T., P.S. Chen, C.H. Wu, Y.T. Tseng, Y.C. Wu, and Y.C. Lo. 2010. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radical Biology & Medicine 49: 1471–9.
LeBel, C.P., H. Ischiropoulos, and S.C. Bondy. 1992. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical Research in Toxicology 5: 227–31.
Erickson, S.E., G.S. Martin, J.L. Davis, M.A. Matthay, and M.D. Eisner. 2009. Recent trends in acute lung injury mortality: 1996–2005. Critical Care Medicine 37: 1574–9.
Randolph, A.G. 2009. Management of acute lung injury and acute respiratory distress syndrome in children. Critical Care Medicine 37: 2448–54.
Folkerts, G., J. Kloek, R.B. Muijsers, and F.P. Nijkamp. 2001. Reactive nitrogen and oxygen species in airway inflammation. European Journal of Pharmacology 429: 251–62.
Votta-Velis, E.G., R.D. Minshall, D.J. Visintine, M. Castellon, and I.V. Balyasnikova. 2007. Propofol attenuates endotoxin-induced endothelial cell injury, angiotensin-converting enzyme shedding, and lung edema. Anesthesia & Analgesia 105: 1363–70. table of contents.
Yeh, C.H., W. Cho, E.C. So, et al. 2011. Propofol inhibits lipopolysaccharide-induced lung epithelial cell injury by reducing hypoxia-inducible factor-1alpha expression. British Journal of Anaesthesia 106: 590–9.
Zou, X., Z. Feng, Y. Li, et al. 2012. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. The Journal of Nutritional Biochemistry 23: 994–1006.
Galani, V., E. Tatsaki, M. Bai, et al. 2010. The role of apoptosis in the pathophysiology of acute respiratory distress syndrome (ARDS): an up-to-date cell-specific review. Pathology-Research and Practice 206: 145–50.
Asehnoune, K., D. Strassheim, S. Mitra, J.Y. Kim, and E. Abraham. 2004. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF–kappa B. The Journal of Immunology 172: 2522–9.
Hsing, C.H., M.C. Lin, P.C. Choi, et al. 2011. Anesthetic propofol reduces endotoxic inflammation by inhibiting reactive oxygen species-regulated Akt/IKKbeta/NF–kappaB signaling. PloS One 6: e17598.
Ko, H.M., S.Y. Kim, S.H. Joo, et al. 2013. Synergistic activation of lipopolysaccharide-stimulated glial cells by propofol. Biochemical and Biophysical Research Communications 438: 420–6.
Mitsuishi, Y., H. Motohashi, and M. Yamamoto. 2012. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Frontiers in Oncology 2: 200.
Garib, V., K. Lang, B. Niggemann, K.S. Zanker, L. Brandt, and T. Dittmar. 2005. Propofol-induced calcium signalling and actin reorganization within breast carcinoma cells. Eur J Anaesthesiology 22: 609–15.
Zhang, L., N. Wang, S. Zhou, W. Ye, G. Jing, and M. Zhang. 2012. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. Journal of Experimental & Clinical Cancer Research 31: 66.
Mammoto, T., M. Mukai, A. Mammoto, et al. 2002. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Letters 184: 165–70.
Wu, K.C., S.T. Yang, T.C. Hsia, et al. 2012. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Research 32: 4833–42.
Acknowledgments
This study was supported by grants provided by Kaohsiung Medical University [grant number: M110022] and the National Science Council of Taiwan to Y.C.L. [grant number: NSC 102-2628-B-037-001-MY3].
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper. The authors report no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, HT., Tseng, YT., Hsu, YY. et al. Propofol Attenuates Lipopolysaccharide-Induced Reactive Oxygen Species Production Through Activation of Nrf2/GSH and Suppression of NADPH Oxidase in Human Alveolar Epithelial Cells. Inflammation 38, 415–423 (2015). https://doi.org/10.1007/s10753-014-0046-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0046-4