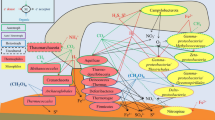
Abstract
Gypsum crusts containing multicolored, stratified microbial communities develop in the evaporation ponds of a commercial saltern in Eilat, Israel at salt concentrations between 190 and 240 g l−1. The upper 0.5–2 cm of the crust is densely populated by orange-brown unicellular cyanobacteria. Below, a layer of green-colored filamentous cyanobacteria is found. Underneath, a bright purple layer of anoxygenic phototrophs is present, below which a reduced black layer is found. We have investigated the biological properties of this crust using a wide variety of techniques, and we here review the results of these interdisciplinary studies. The tests performed included microscopic examination of the biota, phylogenetic analyses based on 16S rRNA gene clone libraries and denaturing gradient gel electrophoresis, fatty acid analysis, light intensity and light quality measurements, microelectrode studies of oxygen profiles and oxygen evolution, determination of sulfate reduction using radioisotope methods, and measurement of methane evolution. The stable vertical stratification in the system enabled separate analyses of the different layers with a high spatial resolution. It was therefore possible to combine the different approaches and obtain information on the activities of the different types of oxygenic and anoxygenic phototrophs, dissimilatory sulfate reducers and methanogens in the different layers, as well as phylogenetic information on the nature of the microorganisms responsible for these processes. The gypsum crust thus becomes a paradigm for the study of a wide variety of microbial processes and their interrelationships in the presence of high salt concentrations.

Similar content being viewed by others
References
Airs, R. L. & B. J. Keely, 2003. A high resolution study of the chlorophyll and bacteriochlorophyll pigment distributions in a calcite/gypsum microbial mat. Organic Geochemistry 34: 539–551.
Antón, J., A. Oren, S. Benlloch, F. Rodríguez-Valera, R. Amann & R. Rosselló-Mora, 2002. Salinibacter ruber gen. nov., sp. nov., a novel extremely halophilic member of the Bacteria from saltern crystallizer ponds. International Journal of Systematic and Evolutionary Microbiology 52: 485–491.
Bolhuis, H., E. M. te Poele & F. Rodríguez-Valera, 2004. Isolation and cultivation of Walsby’s square archaeon. Environmental Microbiology 6: 1287–1291.
Boone, D. R., 2001. Genus IV: Methanohalophilus. In Boone, D. R. & R. W. Castenholz (eds), Bergey’s Manual of Systematic Bacteriology, 2nd Edition. Volume 1. The Archaea and the Deeply Branching and Phototrophic Bacteria. Springer, New York: 281–283.
Brandt, K. K. & K. Ingvorsen, 1997. Desulfobacter halotolerans sp. nov., a halotolerant acetate-oxidizing sulfate-reducing bacterium isolated from sediments of Great Salt Lake, Utah. Systematic and Applied Microbiology 20: 366–373.
Brandt, K. K., F. Vester, A. N. Jensen & K. Ingvorsen, 2001. Sulfate reduction dynamics and enumeration of sulfate-reducing bacteria in hypersaline sediments of the Great Salt Lake. Microbial Ecology 41: 1–11.
Burns, D. G., H. M. Camakaris, P. H. Janssen & M. L. Dyall-Smith, 2004. Cultivation of Walsby’s square haloarchaeon. FEMS Microbiology Letters 238: 469–473.
Burns, D. G., P. H. Janssen, T. Itoh, M. Kamekura, Z. Li, G. Jensen, F. Rodríguez-Valera, H. Bolhuis & M. L. Dyall-Smith, 2007. Haloquadratum walsbyi gen nov., sp. nov., the square haloarchaeon of Walsby, isolated from saltern crystallizers in Australia and Spain. International Journal of Systematic and Evolutionary Microbiology 57: 387–392.
Canfield, D. E., K. B. Sørensen & A. Oren, 2004. Biogeochemistry of a gypsum-encrusted microbial ecosystem. Geobiology 2: 133–150.
Caumette, P., 1993. Ecology and physiology of phototrophic bacteria and sulfate-reducing bacteria in marine salterns. Experientia 49: 473–486.
Caumette, P., R. Baulaigue & R. Matheron, 1988. Characterization of Chromatium salexigens sp. nov., a halophilic Chromatiaceae isolated from Mediterranean salterns. Systematic and Applied Microbiology 10: 284–292.
Caumette, P., R. Matheron, N. Raymond & J.-C. Relexans, 1994. Microbial mats in the hypersaline ponds of Mediterranean salterns (Salins-de-Giraud, France). FEMS Microbiology Ecology 13: 273–286.
Cohen, Y., E. Padan & M. Shilo, 1975. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. Journal of Bacteriology 123: 855–861.
Conrad, R., P. Frenzel & Y. Cohen, 1995. Methane emission from hypersaline microbial mats: lack of aerobic methane oxidation activity. FEMS Microbiology Ecology 16: 297–306.
Cornée, A., 1984. Étude préliminaire des bactéries des saumures et des sédiments des salins de Santa Pola (Espagne). Comparison avec les marais salants de Salin-de-Giraud (Sud de la France). Revista d’Investigacions Geologiques 38/39: 109–122.
Elevi Bardavid, R., D. Ionescu, A. Oren, F. A. Rainey, B. J. Hollen, D. R. Bagaley, A. M. Small & C. M. McKay, 2007. Selective enrichment, isolation and molecular detection of Salinibacter and related extremely halophilic Bacteria from hypersaline environments. Hydrobiologia 576: 3–13.
Fossing, H. & B. B. Jørgensen, 1989. Measurement of bacterial sulfate reduction in sediments. Evaluation of a single-step chromium reduction method. Biogeochemistry 8: 205–222.
Garcia-Pichel, F., U. Nübel & G. Muyzer, 1998. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Archives of Microbiology 169: 469–482.
Grimalt, J. O., R. de Wit, P. Teixidor & J. Albaiges, 1992. Lipid biogeochemistry of Phormidium and Microcoleus mats. Organic Geochemistry 19: 509–530.
Ionescu, D., A. Lipski, K. Altendorf & A. Oren, 2007. Characterization of the endoevaporitic microbial communities in a hypersaline gypsum crust by fatty acid analysis. Hydrobiologia 576: 15–26.
Jahnke, L. L., B. Lee, M. J. Sweeney & H. P. Klein, 1989. Anaerobic biosynthesis of unsaturated fatty acids in the cyanobacterium, Oscillatoria limnetica. Archives of Microbiology 152: 215–217.
Javor, B., 1989. Hypersaline Environments. Microbiology and Biogeochemistry. Springer-Verlag, Berlin.
Jonkers, H. M., R. Ludwig, R. de Wit, O. Pringault, G. Muyzer, H. Niemann, N. Finke & D. de Beer, 2003. Structural and functional analysis of a microbial mat ecosystem from a unique permanent hypersaline inland lake: ‘La Salada de Chiprana’ (NE Spain). FEMS Microbiology Ecology 44: 175–189.
Kedar, L., Y. Kashman & A. Oren, 2002. Mycosporine-2-glycine is the major mycosporine-like amino acid in a unicellular cyanobacterium (Euhalothece sp.) isolated from a gypsum crust in a hypersaline saltern pond. FEMS Microbiology Letters 208: 233–237.
Klappenbach, J. A. & B. K. Pierson, 2004. Phylogenetic and physiological characterization of a filamentous anoxygenic photoautotrophic bacterium ‘Candidatus Chlorothrix halophila’ gen. nov., sp. nov., recovered from hypersaline microbial mats. Archives of Microbiology 181: 17–25.
Lassen, C., H. Plough & B. B. Jørgensen, 1992. A fibre-optic scalar irradiance microsensor: application for spectral light measurements in sediments. FEMS Microbiology Ecology 86: 247–254.
Margheri, M. C., M. R. Tredici, L. Barsanti & W. Balloni, 1987. The photosynthetic community of the Trapani saline lagoons: an alternative option for the exploitation of an extreme environment. Annali di Microbiologia ed Enzimologia 37: 203–215.
Mouné, S., P. Caumette, R. Matheron & J. Willison, 2003. Molecular sequence analysis of prokaryotic diversity in the anoxic sediments underlying cyanobacterial mats of two hypersaline ponds in Mediterranean salterns. FEMS Microbiology Ecology 44: 117–130.
Nübel, U., F. Garcia-Pichel & G. Muyzer, 2000a. The halotolerance and phylogeny of cyanobacteria with tightly coiled trichomes (Spirulina Turpin) and the description of Halospirulina tapeticola gen. nov., sp. nov. International Journal of Systematic and Evolutionary Microbiology 50: 1265–1277.
Nübel, U., F. Garcia-Pichel, E. Clavero & G. Muyzer, 2000b. Matching molecular diversity and ecophysiology of benthic cyanobacteria and diatoms in communities along a salinity gradient. Environmental Microbiology 2: 217–226.
Nübel, U., M. M. Bateson, M. T. Madigan, M. Kühl & D. M. Ward, 2001. Diversity and distribution in hypersaline microbial mats of bacteria related to Chloroflexus spp. Applied and Environmental Microbiology 67: 4365–4371.
Ollivier, B., M.-L. Fardeau, J.-L. Cayol, M. Magot, B. K. C. Patel, G. Prensier & J.-L. Garcia, 1998. Methanocalculus halotolerans gen. nov., sp. nov., isolated from an oil-producing well. International Journal of Systematic Bacteriology 48: 821–828.
Oren, A., 1997. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiology Journal 14: 233–242.
Oren, A., 1999. Bioenergetic aspects of halophilism. Microbiology and Molecular Biology Reviews 63: 334–348.
Oren, A., 2000. Salts and brines. In Whitton, B. A. & M. Potts (eds), Ecology of Cyanobacteria: Their Diversity in Time and Space. Kluwer, Dordrecht: 281–306.
Oren, A., 2002. Halophilic Microorganisms and their Environments. Kluwer, Dordrecht.
Oren, A., 2005. Microscopic examination of microbial communities along a salinity gradient in saltern evaporation ponds: a ‘halophilic safari’. In Gunde-Cimerman, N., A. Oren & A. Plemenitaš (eds), Adaptation to Life at High Salt Concentrations in Archaea, Bacteria. and Eukarya. Springer, Dordrecht: 41–57.
Oren, A., 2006. Life at high salt concentrations. In Dworkin, M., S. Falkow, E. Rosenberg, K.-H. Schleifer & E. Stackebrandt (eds), The Prokaryotes: A handbook on the Biology of Bacteria: Ecophysiology and Biochemistry, Vol. 2. Springer, New York: 263–282.
Oren, A., M. Kühl & U. Karsten, 1995. An endevaporitic microbial mat within a gypsum crust: zonation of phototrophs, photopigments, and light penetration. Marine Ecology Progress Series 128: 151–159.
Oren, A., D. Ionescu, A. Lipski & K. Altendorf, 2005. Fatty acid analysis of a layered community of cyanobacteria developing in a hypersaline gypsum crust. Algological Studies 117: 1–9.
Pierson, B. K., D. Valdez, M. Larsen, E. Morgan & E. E. Mack, 1994. Chloroflexus-like organisms from marine and hypersaline environments: distribution and diversity. Photosynthesis Research 41: 35–52.
Revsbech, N. P., 1989. An oxygen microsensor with a guard cathode. Limnology and Oceanography 34: 474–478.
Revsbech, N. P., B. B. Jørgensen & O. Brix, 1981. Primary production of microalgae in sediments measured by oxygen microprofile, H14CO3 − fixation, and oxygen exchange methods. Limnology and Oceanography 26: 717–730.
Revsbech, N. P., B. B. Jørgensen & T. H. Blackburn, 1983. Microelectrode studies of the photosynthesis and O2, H2S, and pH profiles of a microbial mat. Limnology and Oceanography 28: 1062–1074.
Rothschild, L. J., L. J. Giver, M. R. White & R. L. Mancinelli, 1994. Metabolic activity of microorganisms in evaporites. Journal of Phycology 30: 431–438.
Sørensen, K. B., D. E. Canfield & A. Oren, 2004. Salt responses of benthic microbial communities in a solar saltern (Eilat, Israel). Applied and Environmental Microbiology 70: 1608–1616.
Sørensen, K. B., D. E. Canfield, A. P. Teske & A. Oren, 2005. Community composition of a hypersaline endoevaporitic microbial mat (Israel). Applied and Environmental Microbiology 71: 7352–7365.
Spear, J. R., R. E. Ley, A. B. Berger & N. E. Pace, 2003. Complexity in natural microbial ecosystems: the Guerrero Negro experience. Biological Bulletin 204: 168–173.
Teske, A., K.-U. Hinrichs, V. Edgcomb, A. de Vera Gomez, D. Kysela, S. P. Sylva, M. L. Sogin & H. W. Jannasch, 2002. Microbial diversity in hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Applied and Environmental Microbiology 68: 1994–2007.
van der Wielen, P. W. J. J., H. Bolhuis, S. Borin, D. Daffonchio, C. Corselli, L. Giuliano, G. D’Auria, G. J. de Lange, A. Huebner, S. P. Varnavas, J. Thomson, C. Tamburini, D. Marty, T. J. McGenity, K. N. Timmis & BioDeep Scientific Party, 2005. The enigma of prokaryotic life in deep hypersaline anoxic basins. Science 307: 121–123.
Volkmann, M., A. A. Gorbushina, L. Kedar & A. Oren, 2006. The structure of euhalothece-362, a novel red-shifted mycosporine-like amino acid, from a halophilic cyanobacterium (Euhalothece sp.). FEMS Microbiology Letters 258: 50–54.
Acknowledgments
We thank the Israel Salt Company in Eilat, Israel for allowing access to the salterns, and the staff of the Interuniversity Institute for Marine Sciences of Eilat and the Moshe Shilo Minerva Center for Marine Biogeochemistry for logistic support. Different aspects of this research project were financially supported by the Danish Basic Research Foundation (Grundforskningsfonden), the Danish Research Agency (Statens Naturvidenskablige Forskningsråd), the Israel Science Foundation founded by the Israel Academy of Sciences and Humanities, the NASA Astrobiology Institutes “Subsurface Biospheres” and “Environmental Genomics”, and the State of Lower-Saxony and the Volkswagen Foundation, Hannover, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: J. John & B. Timms
Salt Lake Research: Biodiversity and Conservation—Selected papers from the 9th Conference of the International Society for Salt Lake Research
Rights and permissions
About this article
Cite this article
Oren, A., Sørensen, K.B., Canfield, D.E. et al. Microbial communities and processes within a hypersaline gypsum crust in a saltern evaporation pond (Eilat, Israel). Hydrobiologia 626, 15–26 (2009). https://doi.org/10.1007/s10750-009-9734-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9734-8