Abstract
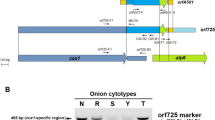
Cytoplasmic male-sterility (CMS) has been used in F1 hybrid seed production in onion (Allium cepa L.). Two types of CMS (CMS-S and CMS-T) have been reported in onions. Almost complete mitochondrial genome sequences of normal, CMS-T, and CMS-S cytoplasm types have been obtained in previous studies. Unlike highly divergent CMS-S mitochondrial genome, there were only three single nucleotide polymorphisms (SNPs) between normal and CMS-T mitochondrial genomes except for orf725, the causal gene for CMS induction. Cleaved amplified polymorphic sequence (CAPS) and high resolution melting (HRM) markers were developed based on one of these SNPs. When these markers were tested for 243 diverse breeding lines, four of them containing CMS-T cytoplasm showed SNP genotypes of the normal cytoplasm. Although mitochondrial genome organizations of these four accessions were assumed to be similar to those of normal and CMS-T, the copy number of orf725 was less than half of that of general CMS-T, suggesting that male-sterility of this variant of CMS-T might be induced by an independent event of substoichiometric shifting of orf725. To distinguish normal and all types of CMS cytoplasms, an HRM marker was developed on the basis of cox1 and orf725. In addition, for genotyping the Ms locus, a nuclear restorer-of-fertility (Rf) gene, approximately 15 kb full-length genomic DNA sequences of AcPMS1, a candidate Rf gene, were obtained. Two reproducible HRM markers were developed among ten candidate HRM markers designed on the basis of more than 800 SNPs or InDels between AcPMS1 alleles.





Similar content being viewed by others
References
Abdelnoor RV, Christensen AC, Mohammed S, Munoz-Castillo B, Moriyama H, Mackenzie SA (2006) Mitochondrial genome dynamics in plants and animals: convergent gene fusions of a MutS homologue. J Mol Evol 63:165–173
Albert B, Godelle B, Gouyon PH (1998) Evolution of the plant mitochondrial genome: dynamics of duplication and deletion of sequences. J Mol Evol 46:155–158
Allen JO, Fauron CM, Mink P, Roark L, Oddiraju S, Lin GN, Meyer L, Sun H, Kim K, Wang C, Du F, Xu D, Gibson M, Cifrese J, Clifton SW, Newton KJ (2007) Comparisons among two fertile and three male-sterile mitochondrial genomes of maize. Genetics 177:1173–1192
Arrieta-Montiel M, Lyznik A, Woloszynska M, Janska H, Tohme J, Mackenzie SA (2001) Tracing evolutionary and developmental implications of mitochondrial stoichiometric shifting in the common bean. Genetics 158:851–864
Backert S, Neilsen BL, Börner T (1997) The mystery of the rings: structure and replication of mitochondrial genomes from higher plants. Trends Plant Sci 2:477–483
Bellaoui M, Martin-Canadell A, Pelletier G, Budar F (1998) Low-copy-number molecules are produced by recombination, actively maintained and can be amplified in the mitochondrial genome of Brassicaceae: relationship to reversion of the male sterile phenotype in some cybrids. Mol Gen Genet 257:177–185
Berninger E (1965) Contribution à l’étude de la sterilité mâle de l’oignon (Allium cepa L.). Ann Amélior Plant 15:183–199
Bohra A, Jha UC, Adhimoolam P, Bisht D, Singh NP (2016) Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep 35:967–993
Budar F, Touzet P, De Paepe R (2003) The nucleo-mitochondrial conflict in cytoplasmic male sterilities revised. Genetica 117:3–16
Cebeci E, Hanci F (2016) Male sterility applications in Allium. Acta Hortic 1145:51–55
Chen L, Liu YG (2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65:579–606
Colombo N, Galmarini CR (2017) The use of genetic, manual and chemical methods to control pollination in vegetable hybrid seed production: a review. Plant Breed 136:287–299
Cui X, Wise RP, Schnable PS (1996) The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 272:1334–1336
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Engelke T, Terefe D, Tatlioglu T (2003) A PCR-based marker system monitoring CMS-(S), CMS-(T) and (N)-cytoplasm in the onion (Allium cepa L.). Theor Appl Genet 107:162–167
Fujii S, Toriyama K (2009) Suppressed expression of retrograde-regulated male sterility restores pollen fertility in cytoplasmic male sterile rice plants. Proc Natl Acad Sci USA 106:9513–9518
Gaborieau L, Brown GG, Mireau H (2016) The propensity of pentatricopeptide repeat genes to evolve into restorers of cytoplasmic male sterility. Front Plant Sci 7:1816
Gökçe AF, Havey MJ (2002) Linkage equilibrium among tightly linked RFLPs and the Ms locus in open-pollinated onion populations. J Am Soc Hortic Sci 127:944–946
Griffiths G, Trueman L, Crowther T, Thomas B, Smith B (2002) Onions-A global benefit to health. Phytother Res 16:603–615
Hanson MR, Bentolila S (2004) Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16:S154–S169
Havey MJ (1995) Identification of cytoplasms using the polymerase chain reaction to aid in the extraction of maintainer lines from open-pollinated populations of onion. Theor Appl Genet 90:263–268
Havey MJ (2013) Single nucleotide polymorphisms in linkage disequilibrium with the male-sterility restoration (Ms) locus in open-pollinated and inbred populations of onion. J Am Soc Hortic Sci 138:306–309
Hu J, Wang K, Huang W, Liu G, Gao Y, Wang J, Huang Q, Ji Y, Qin X, Wan L, Zhu R, Li S, Yang D, Zhu Y (2012) The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant Cell 24:109–122
Janska H, Sarria R, Woloszynska M, Arrieta-Montiel M, Mackenzie SA (1998) Stoichiometric shifts in the common bean mitochondrial genome leading to male sterility and spontaneous reversion to fertility. Plant Cell 10:1163–1180
Jones HA, Clarke A (1943) Inheritance of male sterility in the onion and the production of hybrid seed. Proc Am Soc Hortic Sci 43:189–194
Jones HA, Emsweller SL (1936) A male-sterile onion. Proc Am Soc Hortic Sci 34:582–585
Khrustaleva L, Jiang J, Havey MJ (2016) High-resolution tyramide-FISH mapping of markers tightly linked to the male-fertility restoration (Ms) locus of onion. Theor Appl Genet 129:535–545
Kim S (2014) A codominant molecular marker in linkage disequilibrium with a restorer-of-fertility gene (Ms) and its application in reevaluation of inheritance of fertility restoration in onions. Mol Breed 34:769–778
Kim Y, Zhang D (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23:53–65
Kim S, Lim H, Park S, Cho K, Sung S, Oh D, Kim K (2007) Identification of a novel mitochondrial genome type and development of molecular makers for cytoplasm classification in radish (Raphanus sativus L.). Theor Appl Genet 115:1137–1145
Kim S, Lee E, Cho DY, Han T, Bang H, Patil BS, Ahn YK, Yoon M (2009) Identification of a novel chimeric gene, orf725, and its use in development of a molecular marker for distinguishing three cytoplasm types in onion (Allium cepa L.). Theor Appl Genet 118:433–441
Kim S, Kim C, Park M, Choi D (2015) Identification of candidate genes associated with fertility restoration of cytoplasmic male-sterility in onion (Allium cepa L.) using a combination of bulked segregant analysis and RNA-seq. Theor Appl Genet 128:2289–2299
Kim B, Kim K, Yang T, Kim S (2016) Completion of the mitochondrial genome sequence of onion (Allium cepa L.) containing the CMS-S male-sterile cytoplasm and identification of an independent event of the ccmF N gene split. Curr Genet 62:873–885
Kim B, Kim C, Kim S (2019a) Inheritance of fertility restoration of male-sterility conferred by cytotype Y and identification of instability of male fertility phenotypes in onion (Allium cepa L.). J Hortic Sci Biotechnol 94:341–348
Kim B, Yang T, Kim S (2019b) Identification of a gene responsible for cytoplasmic male-sterility in onions (Allium cepa L.) using comparative analysis of mitochondrial genome sequences of two recently diverged cytoplasms. Theor Appl Genet 132:313–322
Kitazaki K, Arakawa T, Matsunaga M, Yui-Kurino R, Matsuhira H, Mikami T, Kubo T (2015) Post-translational mechanisms are associated with fertility restoration of cytoplasmic male sterility in sugar beet (Beta vulgaris). Plant J 83:290–299
Kmiec B, Woloszynska M, Janska H (2006) Heteroplasmy as a common state of mitochondrial genetic information in plants and animals. Curr Genet 50:149–159
Laser KD, Lersten NR (1972) Anatomy and cytology of microsporogenesis in cytoplasmic male sterile angiosperms. Bot Rev 38:425–454
Oldenburg DJ, Bendich AJ (2001) Mitochondrial DNA from the Liverwort Marchantia polymorpha: circularly permuted linear molecules, head-to-tail concatemers, and a 5′ protein. J Mol Biol 310:549–562
Rasheed A, Hao Y, Xia X, Khan A, Xu Y, Varshney RK, He Z (2017) Crop breeding chips and genotyping platforms: progress, challenges, and perspectives. Mol Plant 10:1047–1064
Sakai T, Imamura J (1993) Evidence for a mitochondrial sub-genome containing radish AtpA in a Brassica napus cybrid. Plant Sci 90:95–103
Sandhu AP, Abdelnoor RV, Mackenzie SA (2007) Transgenic induction of mitochondrial rearrangements for cytoplasmic male sterility in crop plants. Proc Natl Acad Sci USA 104:1766–1770
Sato Y (1998) PCR amplification of CMS-specific mitochondrial nucleotide sequences to identify cytoplasmic genotypes of onion (Allium cepa L.). Theor Appl Genet 96:367–370
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Schweisguth B (1973) Étude d’un nouveau type de stérilité male chez l’oignon, Allium cepa L. Ann Amélior Plant 23:221–233
Skippington E, Barkman TJ, Rice DW, Palmer JD (2015) Miniaturized mitogenome of the parasitic plant Viscum scurruloideum is extremely divergent and dynamic and has lost all nad genes. Proc Natl Acad Sci USA 112:E3515–3524
Sloan DB (2013) One ring to rule them all? Genome sequencing provides new insights into the 'master circle' model of plant mitochondrial DNA structure. New Phytol 200:978–985
Sloan DB, Alverson AJ, Chuckalovcak JP, Wu M, McCauley DE, Palmer JD, Taylor DR (2012) Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol 10:e1001241
Small I, Suffolk R, Leaver CJ (1989) Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 58:69–76
Soto VC, Caselles CA, Silva MF, Galmarini CR (2018) Onion hybrid seed production: relation with nectar composition and flower traits. J Econ Entomol 111:1023–1029
Woloszynska M, Trojanowski D (2009) Counting mtDNA molecules in Phaseolus vulgaaris: sublimons are constantly produced by recombination via short repeats and undergo rigorous selection during substoichiometric shifting. Plant Mol Biol 70:511–521
Yang YY, Huo YM, Miao J, Liu BJ, Kong SP, Gao LM, Liu C, Wang ZB, Tahara Y, Kitano H, Wu X (2013) Identification of two SCAR markers co-segregated with the dominant Ms and recessive ms alleles in onion (Allium cepa L.). Euphytica 190:267–277
Acknowledgements
This research was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Agriculture, Food and Rural Affairs Research Center Support Program (Vegetable Breeding Research Center) funded by the Ministry of Agriculture, Food and Rural Affairs (710011-03), Golden Seed Project (Center for Horticultural Seed Development, No. 213007-05-3-SBB10), and a grant from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center No. PJ013400). The authors thank Ji-wha Hur, Jeong-Ahn Yoo, and Su-jung Kim for their dedicated technical assistance.
Author information
Authors and Affiliations
Contributions
BK performed most experiments and drafted the manuscript. SK organized and coordinated this research project and edited the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that all experiments complied with current laws of Republic of Korea.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
CAPS and HRM markers designed on the basis of a single SNP between normal and CMS-inducing cytoplasms. A. PCR products of the CAPS marker digested with a Dra I restriction enzyme. B. Normalized melting peak patterns of the HRM marker (TIFF 233 kb)
Supplementary Fig. 2
PCR patterns of three previously reported molecular markers developed for distinguishing onion cytoplasm types. N: normal; T: CMS-T; S: CMS-S. PCR amplifications were carried out according to respective references (TIFF 106 kb)
Supplementary Fig. 3
Normalized melting peak patterns of 10 HRM markers designed on the basis of AcPMS1 genomic DNA polymorphisms. Position of each marker is shown in Fig. 5A (TIFF 1521 kb)
Rights and permissions
About this article
Cite this article
Kim, B., Kim, S. Identification of a variant of CMS-T cytoplasm and development of high resolution melting markers for distinguishing cytoplasm types and genotyping a restorer-of-fertility locus in onion (Allium cepa L.). Euphytica 215, 164 (2019). https://doi.org/10.1007/s10681-019-2492-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-019-2492-4