Abstract
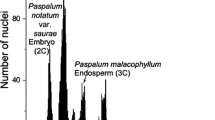
Because of its high biomass productivity and nutrient-use efficiency, Miscanthus spp. have emerged as promising bioenergy crops. Polyploidism in Miscanthus species is important because it allows non-native countries to cultivate sterile types, such as triploid M. × giganteus, in order to prevent ecosystem disturbance. Although M. sacchariflorus and M. sinensis are considered to be native to Korea, China, and Japan, accurate information describing the ploidy levels of these species has not yet been well established. To evaluate rough ploidy levels, 215 accessions of Miscanthus species were estimated by relative fluorescence intensities of DAPI-stained nuclei. After evaluation of rough ploidy levels, 20 plants were randomly selected by species and ploidy levels, and ploidy levels were examined by counting chromosomes, estimating propidium iodide-stained DNA content with flow cytometry, and measuring sizes of stomata and caryopsis. Among the 20 plants examined, 3 were diploid and 6 were tetraploid M. sacchariflorus (4.56 ± 0.01 and 8.90 ± 0.14 pg/2C nuclear DNA content, respectively), while 6 were diploid and 3 were triploid M. sinensis (5.40 ± 0.18 and 8.29 ± 0.33 pg/2C nuclear DNA content, respectively). One plant was a putative triploid M. × giganteus having 7.31 pg/2C nuclear DNA content, which was similar to an M. × giganteus plant from Illinois, USA having 7.23 pg/2C nuclear DNA content. We confirmed the occurrence of diploid and tetraploid M. sacchariflorus and M. sinensis plants as well as a putative triploid M. × giganteus native to Korea. Thus, measurement of the size of stomata and caryopsis allowed prediction of ploidy levels in the field and laboratory.

Similar content being viewed by others
References
Adati S, Shiotani I (1962) The cytotaxonomy of the genus Miscanthus and its phylogenic status. Bull Fac Agric Mie Univ 25:1–14
Ahonsi MO, Agindotan BO, Williams DW, Arundale R, Gray ME, Voigt TB, Bradley CA (2010) First report of Pithomyces chartarum causing a leaf blight of Miscanthus × giganteus in Kentucky. Plant Dis 94:480
Baack EJ (2004) Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus Ranunculaceae). Am J Bot 91:1783–1788
Barney JN, DiTomaso JM (2008) Nonnative species and bioenergy: are we cultivating the next invader? Bioscience 58:64–70
Beaulieu JM, Leitch IJ, Patel S, Pendharker A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179:975–986
Beccari G, Covarelli L, Balmas V, Tosi L (2010) First report of Miscanthus × giganteus rhizome rot caused by Fusarium avenaceum, Fusarium oxysporum, and Muhor hiemalis. Australas Plant Dis Notes 5:28–29
Chen S, Stephen AR (2006) Miscanthus Andersson, Öfvers. Kongl. Vetensk.-Akad. Förh. Flora China 22:581–583
Clifton-Brown JC, Lewandowski I, Andersson B, Basch G, Christian DG, Kjeldsen JB, Jørgensen U, Mortensen JV et al (2001) Performance of 15 Miscanthus genotypes at five sites in Europe. Agron J 93:1013–1019
Clifton-Brown J, Chiang YC, Hodkinson TR (2008) Miscanthus: genetic resources and breeding potential to enhance bioenergy production. In: Booth E, Green M, Karp A, Shield I, Stock D, Turley D (eds) Association of applied biologists aspects of applied biology. Biomass and Energy Crops III, Warwick, pp 273–294
Deuter M (2000) Breeding approaches to improvement of yield and quality in Miscanthus grown in Europe. In: Lewandowski I, Clifton-Brown JC (eds) European Miscanthus improvement-final report September 2000. Institute of Crop Production and Grassland Research, University of Hohenheim, Stuttgart, pp 28–52
Deuter M, Abraham J (1998) Genetic resources of Miscanthus and their use in breeding. In: Kopetz H, Weber T, Palz W, Chartier P, Ferrero GL (eds) Biomass for energy and industry. CARMEN Publisher, Rimpar, pp 775–777
Glowacka K, Jezowski S, Kaczmarek Z (2009) Polyploidization of Miscanthus sinensis and Miscanthus × giganteus by plant colchicine treatment. Ind Crops Prod 30:444–446
Glowacka K, Jezowski S, Kaczmarek Z (2010) In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind Crops Prod 32:88–96
Greef JM, Deuter M, Jung C, Schondelmaier J (1997) Genetic diversity of European Miscanthus species revealed by AFLP fingerprinting. Genet Resour Crop Evol 44:185–195
Hasnain R, Khan MM, Khan AA (2003) Seedlessness in Citrus. Int J Agric Biol 5:388–391
Hignight K, Rush D (2008) Tetraploid perennial ryegrass variety T3. United States Patent (Patent No.: US 7,368,638 B2)
Hirayoshi I, Nishikawa K, Kubono M, Murase T (1957) Cyto-genetical studies on forage plants (VI). On the chromosome number of ogi (Miscanthus sacchariflorus). Res Bull Fac Agr Gifu Univ 8:8–13 [in Japanese with English summary]
Hiroyoshi I, Takashi K, Yoshihiko T (2004) Genetic structure of Miscanthus sinensis ssp. condensatus (Poaceae) on Miyake Island: implications for revegetation of volcanically devastated sites. Ecol Res 20:233–238
Hodgson JG, Sharafi M, Jalili A, Díaz S, Montserrat-Martí G, Palmer C, Cerabolini B, Pierce S et al (2010) Stomatal vs. genome size in angiosperms: the somatic tail wagging the genomic dog? Ann Bot 105:573–584
Hodkinson TR, Chase MW, Takahashi C, Leitch IJ, Bennett MD, Renvoize SA (2002) The use of DNA sequencing (ITS and trnL-F), AFLF, and fluorescent in situ hybridization to study allopolyploid Miscanthus (Poaceae). Am J Bot 89:279–286
Husband BC (2004) The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol J Linn Soc 82:537–546
Koonin SE (2006) Getting serious about biofuels. Science 311:435
Kula A, Dudziak B, Sliwinska E, Grabowska-Joachimiak A, Stewart A, Golczyk H, Joachimiak AJ (2006) Cytomorphological studies on American and European Phleum commutatum Guad. (Poaceae). Acta Biol Cracov Ser Bot 48:99–108
Lee YN (2000) Miscanthus Anderess. Flora of Korea, pp 1032–1034
Levin D (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Lewandowski I, Clifton-Brown JC, Scurlock JMO, Huisman W (2000) Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 19:209–227
Moon YH, Koo BC, Choi YH, Ahn SH, Bark ST, Cha YL, An GH, Kim JK et al (2010) Development of “Miscanthus” the promising bioenergy crop. Kor J Weed Sci 30:330–399 (in Korean with English abstract)
Nishiwaki A, Mizuguti A, Kuwabara S, Toma Y, Ishigaki G, Miyashita T, Yamada T, Matuura H et al (2011) Discovery of natural Miscanthus (Poaceae) triploid plants in sympatric populations of M. sacchariflorus and M. sinensis in southern Japan. Am J Bot 98:154–159
Parthasarathy N, Ramanujam S, Ramiah K (1935) A tetraploid plant in wild rice (Oriza longistaminata). In: Aiyar S (ed) Proceedings of the Indian Academy of Science, Section B. Bangalore, pp 565–570
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Rayburn AL, Crawford J, Rayburn CM, Juvik JA (2009) Genome size of three Miscanthus species. Plant Mol Biol Rep 27:184–188
Rieseberg LH, Willis JH (2007) Plant speciation. Science 317:910–914
Rounsaville TJ, Touchell DH, Ranney TG (2011) Fertility and reproductive pathways in diploid and triploid Miscanthus sinensis. HortScience 46:1353–1357
Smith KF, Mcfarlane NM, Croft VM, Trigg PJ, Kearney GA (2003) The effects of polyploidy and seed mass on the emergence and early vigour of perennial ryegrass (Lolium perenne L.) cultivars. Aust J Exp Agric 43:481–486
Somerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for lignocellulosic biofuels. Science 329:790–792
Susiacue I, Alvarez JM (1997) Fertility and pollen tube growth in polyploid melons (Cucumis melo L.). Euphytica 93:369–373
Swaminathan K, Alabady MS, Varala K, De Paoli E, Ho I, Rokhsar DS, Arumuganathan AK, Ming R et al (2010) Genomic and small RNA sequencing of Miscanthus × giganteus shows the utility of sorghum as a reference genome sequence for Andropogoneae grasses. Genome Biol 11:R12
Tatum TC, Nunez L, Kushad MM, Rayburn AL (2006) Genome size variation in pumpkin (Cucurbita sp.). Ann Appl Bot 149:145–151
Acknowledgments
The authors would like to thank Dr. Juvik of Illinois University for helpful advice during the preparation of this manuscript. Funding was provided by a Grant from the National Institute of Crop Science, Rural Development Administration.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moon, YH., Cha, YL., Choi, YH. et al. Diversity in ploidy levels and nuclear DNA amounts in Korean Miscanthus species. Euphytica 193, 317–326 (2013). https://doi.org/10.1007/s10681-013-0910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-013-0910-6