Summary
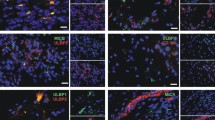
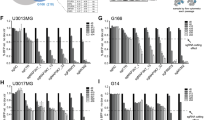
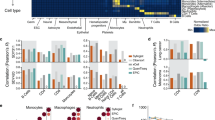
Glioblastoma multiforme (GBM) is the most aggressive human brain tumor, and GBM stem cells (GSC) may be responsible for its recurrence and therapeutic resistance. Toll-like receptors (TLRs), which recognize multiple ligands (endogenous and pathogen-associated) and trigger the immune response of mature immune cells, are also expressed by hematopoietic stem and progenitor cells, where their activation results in the differentiation of these cells into myeloid cells. Since TLR expression has been recently described in neural cells, including neural stem cells, we studied TLR expression by GSCs and the effect of stimulation by TLR ligands on promoting GSC differentiation into mature GBM cells. First, our results showed heterogeneous TLR expression by GBM cells from human tumors and, for the first time, by human GSCs defined by their CD133+ and CD44+ phenotypes. Next, the effect of TLR ligands was studied in in vitro cell cultures of neurospheres and CD44+ cells obtained from two GBM cell lines (U-87 and U-118). The expression of GSC markers diminished in the presence of Pam3CSK4 or LPS (TLR2 and TLR4 ligands, respectively), thus indicating TLR-dependent differentiation. Interestingly, simultaneous treatment with Pam3CSK4 plus temozolomide (TMZ), the reference drug in GBM treatment, significantly increased cell death compared to the effect of the ligand alone, which showed no toxicity, or TMZ alone. These results suggest a synergistic effect between Pam3CSK4 and TMZ based on the induction of TLR-dependent GSC differentiation towards mature GBM cells, which exhibited increased sensitivity to chemotherapy, and provide new perspectives in GBM therapy.





Similar content being viewed by others
References
Holland EC (2000) Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A 97:6242–6244
Damodaran O, van Heerden J, Nowak AK, Bynevelt M, McDonald K, Marsh J, Lee G (2014) Clinical management and survival outcomes of gliosarcomas in the era of multimodality therapy. J Clin Neurosci 21:478–481
Yan K, Yang K, Rich JN (2013) The evolving landscape of glioblastoma stem cells. Curr Opin Neurol 26:701–707
Jackson M, Hassiotou F, Nowak A (2015) Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis 36:177–185
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488:522–526. https://doi.org/10.1038/nature11287
Safari M, Khoshnevisan A (2015) Cancer stem cells and Chemoresistance in glioblastoma multiform: a review article. J Stem Cells 10:271–285
Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K (2015) Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis 2:152–163
Brown DV, Daniel PM, D'Abaco GM, Gogos A, Ng W, Morokoff AP, Mantamadiotis T (2015) Coexpression analysis of CD133 and CD44 identifies proneural and mesenchymal subtypes of glioblastoma multiforme. Oncotarget 20:6267–6280
Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K (2015) Emerging targets for glioblastoma stem cell therapy. J Biomed Res 20(30):19–31. https://doi.org/10.7555/JBR.30.20150100
Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, Rodón L, Folch G, Carmona MA, Prieto-Sánchez RM, Barba I, Martínez-Sáez E, Prudkin L, Cuartas I, Raventós C, Martínez-Ricarte F, Poca MA, García-Dorado D, Lahn MM, Yingling JM, Rodón J, Sahuquillo J, Baselga J, Seoane J (2010) TGF-β receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell 18:655–668
Liu Y, Han SSW, Wu Y, Tuohy TMF, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS (2004) CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol 276:31–46
Yi Y, Hsieh IY, Huang X, Li J, Zhao W (2016) Glioblastoma stem-like cells: characteristics, microenvironment, and Therapy. Front Pharmacol 7:477
Bradshaw A, Wickremsekera A, Tan ST, Peng L, Davis PF, Itinteang T (2016) Cancer stem cell hierarchy in glioblastoma Multiforme. Front Surg 15:21
Wei KC, Huang CY, Chen PY, Feng LY, Wu TW, Chen SM, Tsai HC, Lu YJ, Tsang NM, Tseng CK, Pai PC, Shin JW (2010) Evaluation of the prognostic value of CD44 in glioblastoma multiforme. Anticancer Res 30:253–259
Denysenko T, Gennero L, Roos MA, Melcarne A, Juenemann C, Faccani G, Morra I, Cavallo G, Reguzzi S, Pescarmona G, Ponzetto A (2010) Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct 28:343–351. https://doi.org/10.1002/cbf.1666
Jhanwar-Uniyal M, Labagnara M, Friedman M, Kwasnicki A, Murali R (2015) Glioblastoma: molecular pathways, stem cells and therapeutic targets. Cancers (Basel) 7:538–555
Hueng DY, Sytwu HK, Huang SM, Chang C, Ma HI (2011) Isolation and characterization of tumor stem-like cells from human meningiomas. J Neuro-Oncol 104:45–53
Kawai T, Akira S (2011) The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11:373–384
King KY, Goodell MA (2011) Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol 11:685–692
Yáñez A, Goodridge HS, Gozalbo D, Gil ML (2013) TLRs control hematopoiesis during infection. Eur J Immunol 43:2526–2533. https://doi.org/10.1002/eji.201343833
Yáñez A, Megías J, O'Connor JE, Gozalbo D, Gil ML (2011) Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling. PLoS One 6:e24761
Megías J, Yáñez A, Moriano S, O'Connor JE, Gozalbo D, Gil ML (2012) Direct toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells 30:1486–1495
Megías J, Maneu V, Salvador P, Gozalbo D, Gil ML (2013) Candida albicans stimulates in vivo differentiation of haematopoietic stem and progenitor cells towards macrophages by a TLR2-dependent signalling. Cell Microbiol 15:1143–1153
Lee H, Lee S, Cho IH, Lee SJ (2013) Toll-like receptors: sensor molecules for detecting damage to the nervous system. Curr Protein Pept Sci 14:33–42
Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E (2012) Involvement of TNFα-induced TLR4-NF-κB and TLR4-HIF-1α feed-forward loops in the regulation of inflammatory responses in glioma. J Mol Med (Berl) 90:67–80
Alvarado AG, Thiagarajan PS, Mulkearns-Hubert EE, Silver DJ, Hale JS, Alban TJ, Turaga SM, Jarrar A, Reizes O, Longworth MS, Vogelbaum MA, Lathia JD (2017) Glioblastoma Cancer stem cells evade innate immune suppression of self-renewal through reduced TLR4 expression. Cell Stem Cell 20:450–461.e4. https://doi.org/10.1016/j.stem.2016.12.001
Zeuner M, Bieback K, Widera D (2015) Controversial role of toll-like receptor 4 in adult stem cells. Stem Cell Rev 11:621–634. https://doi.org/10.1007/s12015-015-9589-5
Ulrich H, do Nascimento IC, Bocsi J, Tárnok A (2014) Immunomodulation in stem cell differentiation into neurons and brain repair. Stem Cell Rev 11:474–486. https://doi.org/10.1007/s12015-014-9556-6
Gil-Benso R, Monteagudo C, Cerdá-Nicolás M, Callaghan RC, Pinto S, Martínez-Romero A, Pellín-Carcelén A, San-Miguel T, Cigudosa JC, López-Ginés C (2012) Characterization of a new human melanoma cell line with CD133 expression. Hum Cell 25:61–67
Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN (2015) Cancer stem cells in glioblastoma. Genes Dev 29:1203–1217. https://doi.org/10.1101/gad.261982.115
Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava S, Ravanini M, Facchetti F, Bruzzone MG, Finocchiaro G (2006) Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res 66:10247–10252
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH, Gao L, Wang JM, Bian XW (2008) Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett 265:124–134. https://doi.org/10.1016/j.canlet.2008.02.010
Stopschinski BE, Beier CP, Beier D (2013) Glioblastoma cancer stem cells-from concept to clinical application. Cancer Lett 338:32–40. https://doi.org/10.1016/j.canlet.2012.05.033
Binder ZA, Wilson KM, Salmasi V, Orr BA, Eberhart CG, Siu IM, Lim M, Weingart JD, Quinones-Hinojosa A, Bettegowda C, Kassam AB, Olivi A, Brem H, Riggins GJ, Gallia GL (2016) Establishment and biological characterization of a panel of glioblastoma Multiforme (GBM) and GBM variant Oncosphere cell lines. PLoS One 11:e0150271. https://doi.org/10.1371/journal.pone.0150271
Suslov ON, Kukekov VG, Ignatova TN, Steindler DA (2002) Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci U S A 99:14506–14511
Finocchiaro G (2017) TLRgeting evasion of immune pathways in glioblastoma. Cell Stem Cell 20:422–424. https://doi.org/10.1016/j.stem.2017.03.018
Dragu DL, Necula LG, Bleotu C, Diaconu CC, Chivu-Economescu M (2015) Therapies targeting cancer stem cells: current trends and future challenges. World J Stem Cells 7:1185–1201. https://doi.org/10.4252/wjsc.v7.i9.1185
Maywald O, Buchheidt D, Bergmann J, Schoch C, Ludwig WD, Reiter A, Hastka J, Lengfelder E, Hehlmann R (2004) Spontaneous remission in adult acute myeloid leukemia in association with systemic bacterial infection-case report and review of the literature. Ann Hematol 83:189–194
Ignatz-Hoover JJ, Wang H, Moreton SA, Chakrabarti A, Agarwal MK, Sun K, Gupta K, Wald DN (2015) The role of TLR8 signaling in acute myeloid leukemia differentiation. Leukemia 29:918–926. https://doi.org/10.1038/leu.2014.293
Villamón E, González-Fernández J, Such E, Cervera JV, Gozalbo D, Luisa Gil M (2018) Imiquimod inhibits growth and induces differentiation of myeloid leukemia cell lines. Cancer Cell Int 18:15
Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, Combs SE, Goidts V, Helmke BM, Eckstein V, Roth W, Beckhove P, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende C (2010) Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res 15:2715–2728. https://doi.org/10.1158/1078-0432.CCR-09-1800
Dong Y, Han Q, Zou Y, Deng Z, Lu X, Wang X, Zhang W, Jin H, Su J, Jiang T, Ren H (2012) Long-term exposure to imatinib reduced cancer stem cell ability through induction of cell differentiation via activation of MAPK signaling in glioblastoma cells. Mol Cell Biochem 370:89–102. https://doi.org/10.1007/s11010-012-1401-0
Daniele S, Costa B, Zappelli E, Da Pozzo E, Sestito S, Nesi G, Campiglia P, Marinelli L, Novellino E, Rapposelli S, Martini C (2015) Combined inhibition of AKT/mTOR and MDM2 enhances glioblastoma Multiforme cell apoptosis and differentiation of cancer stem cells. Sci Rep 5:9956. https://doi.org/10.1038/srep09956
Nduom EK, Weller M, Heimberger AB (2015) Immunosuppressive mechanisms in glioblastoma. Neuro Oncol Suppl 7:vii9–vii14. https://doi.org/10.1093/neuonc/nov151
Martínez A, Bono C, Megías J, Yáñez A, Gozalbo D, Gil ML (2017) PRR signaling during in vitro macrophage differentiation from progenitors modulates their subsequent response to inflammatory stimuli. Eur Cytokine Netw 28:102–110. https://doi.org/10.1684/ecn.2017.0398
Megías J, Martínez A, Yáñez A, Goodridge HS, Gozalbo D, Gil ML (2016) TLR2, TLR4 and Dectin-1 signalling in hematopoietic stem and progenitor cells determines the antifungal phenotype of the macrophages they produce. Microbes Infect 18:354–363. https://doi.org/10.1016/j.micinf.2016.01.005
Alexander BM, Galanis E, Yung WK, Ballman KV, Boyett JM, Cloughesy TF, Degroot JF, Huse JT, Mann B, Mason W, Mellinghoff IK, Mikkelsen T, Mischel PS, O'Neill BP, Prados MD, Sarkaria JN, Tawab-Amiri A, Trippa L, Ye X, Ligon KL, Berry DA, Wen PY (2015) Brain malignancy steering committee clinical trials planning workshop: report from the targeted therapies working group. Neuro-Oncology 17:180–188. https://doi.org/10.1093/neuonc/nou154
Prados MD, Byron SA, Tran NL, Phillips JJ, Molinaro AM, Ligon KL, Wen PY, Kuhn JG, Mellinghoff IK, de Groot JF, Colman H, Cloughesy TF, Chang SM, Ryken TC, Tembe WD, Kiefer JA, Berens ME, Craig DW, Carpten JD, Trent JM (2015) Toward precision medicine in glioblastoma: the promise and the challenges. Neuro-Oncology 17:1051–1063. https://doi.org/10.1093/neuonc/nov031
Hussein WM, Liu TY, Skwarczynski M, Toth I (2014) 2014. Expert Opin Ther Pat 24:453–470. https://doi.org/10.1517/13543776.2014.880691
Acknowledgments
This work was supported by the following grants: VLC-Bioclínic-TLR-GBMM-GIL-CERDÁ-2015 from INCLIVA and the University of Valencia, FIS-P114/01669 from the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III) and PROMETEOII/2015/007 from the Generalitat Valenciana. We thank Ms. Ana Clari and Guadalupe Herrera for their technical assistance. The funding sources were not involved in the research and/or preparation of the article.
Funding
The present work was supported by the following grants: VLC-Bioclínic TLR-GBM-GIL-CERDÁ-2015 from INCLIVA and the University of Valencia, FIS-P114/01669 from the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III) and PROMETEOII/2015/007 from the Generalitat Valenciana. The funding sources were not involved in the research and/or preparation of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Javier Megías declares that he has no conflicts of interest. Alba Martínez declares that she has no conflicts of interest. Teresa San-Miguel declares that she has no conflicts of interest. Rosario Gil-Benso declares that she has no conflicts of interest. Lisandra Muñoz-Hidalgo declares that she has no conflicts of interest. David Albert-Bellver declares that he has no conflicts of interest. Amara Carratalá declares that she has no conflicts of interest. Daniel Gozalbo declares that he has no conflicts of interest. Concha López-Ginés declares that she has no conflicts of interest. María Luisa Gil declares that she has no conflicts of interest. Miguel Cerdá-Nicolás declares that he has no conflicts of interest.
Ethical approval
The study was reviewed and approved by the Institutional Ethics Committee of the University of Valencia and Clinic Hospital of Valencia. All procedures performed in this study were in accordance with the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Megías, J., Martínez, A., San-Miguel, T. et al. Pam3CSK4, a TLR2 ligand, induces differentiation of glioblastoma stem cells and confers susceptibility to temozolomide. Invest New Drugs 38, 299–310 (2020). https://doi.org/10.1007/s10637-019-00788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00788-2