Summary
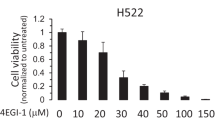
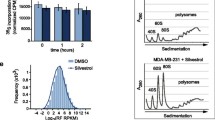
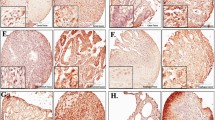
Deregulation of cap-dependent translation has been implicated in the malignant transformation of numerous human tissues. 4EGI-1, a novel small-molecule inhibitor of cap-dependent translation, disrupts formation of the eukaryotic initiation factor 4F (eIF4F) complex. The effects of 4EGI-1-mediated inhibition of translation initiation in malignant pleural mesothelioma (MPM) were examined. 4EGI-1 preferentially inhibited cell viability and induced apoptosis in MPM cells compared to normal mesothelial (LP9) cells. This effect was associated with hypophosphorylation of 4E–binding protein 1 (4E–BP1) and decreased protein levels of the cancer-related genes, c-myc and osteopontin. 4EGI-1 showed enhanced cytotoxicity in combination with pemetrexed or gemcitabine. Translatome-wide polysome microarray analysis revealed a large cohort of genes that were translationally regulated upon treatment with 4EGI-1. The 4EGI-1-regulated translatome was negatively correlated to a previously published translatome regulated by eIF4E overexpression in human mammary epithelial cells, which is in agreement with the notion that 4EGI-1 inhibits the eIF4F complex. These data indicate that inhibition of the eIF4F complex by 4EGI-1 or similar translation inhibitors could be a strategy for treating mesothelioma. Genome wide translational profiling identified a large cohort of promising target genes that should be further evaluated for their potential significance in the treatment of MPM.








Similar content being viewed by others
References
Lazaris-Karatzas A, Montine KS, Sonenberg N (1990) Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 345(6275):544–547
De Benedetti A, Graff JR (2004) eIF-4E expression and its role in malignancies and metastases. Oncogene 23(18):3189–3199
Gingras AC, Raught B, Sonenberg N (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev 15(7):807–826
Pelletier J, Graff J, Ruggero D, Sonenberg N (2015) Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res 75(2):250–263
Graff JR, Zimmer SG (2003) Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis 20(3):265–273
Thumma SC, Kratzke RA (2007) Translational control: a target for cancer therapy. Cancer Lett 258(1):1–8
Rosenwald IB, Chen JJ, Wang S, Savas L, London IM, Pullman J (1999) Upregulation of protein synthesis initiation factor eIF-4E is an early event during colon carcinogenesis. Oncogene 18(15):2507–2517
Kerekatte V, Smiley K, Hu B, Smith A, Gelder F, De Benedetti A (1995) The proto-oncogene/translation factor eIF4E: a survey of its expression in breast carcinomas. Int J Cancer 64(1):27–31
Crew JP, Fuggle S, Bicknell R, Cranston DW, de Benedetti A, Harris AL (2000) Eukaryotic initiation factor-4E in superficial and muscle invasive bladder cancer and its correlation with vascular endothelial growth factor expression and tumour progression. Br J Cancer 82(1):161–166
Nathan CO, Franklin S, Abreo FW, Nassar R, de Benedetti A, Williams J et al (1999) Expression of eIF4E during head and neck tumorigenesis: possible role in angiogenesis. Laryngoscope 109(8):1253–1258
Silvera D, Formenti SC, Schneider RJ (2010) Translational control in cancer. Nat Rev Cancer 10(4):254–266
Bitterman PB, Polunovsky VA (2015) eIF4E-mediated translational control of cancer incidence. Biochim Biophys Acta 1849(7):774–780
Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK (1999) Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell 3(6):707–716
Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A et al (2007) Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 128(2):257–267
Yi T, Kabha E, Papadopoulos E, Wagner G (2014) 4EGI-1 targets breast cancer stem cells by selective inhibition of translation that persists in CSC maintenance, proliferation and metastasis. Oncotarget 5(15):6028–6037
Wang H, Huang F, Wang J, Wang P, Lv W, Hong L et al (2015) The synergistic inhibition of breast cancer proliferation by combined treatment with 4EGI-1 and MK2206. Cell Cycle 14(2):232–242
Willimott S, Beck D, Ahearne MJ, Adams VC, Wagner SD (2013) Cap-translation inhibitor, 4EGI-1, restores sensitivity to ABT-737 apoptosis through cap-dependent and -independent mechanisms in chronic lymphocytic leukemia. Clin Cancer Res 19(12):3212–3223
Descamps G, Gomez-Bougie P, Tamburini J, Green A, Bouscary D, Maiga S et al (2012) The cap-translation inhibitor 4EGI-1 induces apoptosis in multiple myeloma through Noxa induction. Br J Cancer 106(10):1660–1667
Yang X, Dong QF, Li LW, Huo JL, Li PQ, Fei Z et al (2015) The cap-translation inhibitor 4EGI-1 induces mitochondrial dysfunction via regulation of mitochondrial dynamic proteins in human glioma U251 cells. Neurochem Int 90:98–106
Wu M, Zhang C, Li XJ, Liu Q, Wanggou S (2016) Anti-cancer effect of cap-translation inhibitor 4EGI-1 in human Glioma U87 cells: involvement of mitochondrial dysfunction and ER stress. Cell Physiol Biochem 40(5):1013–1028
Chen L, Aktas BH, Wang Y, He X, Sahoo R, Zhang N et al (2012) Tumor suppression by small molecule inhibitors of translation initiation. Oncotarget 3(8):869–881
Schwarzer A, Holtmann H, Brugman M, Meyer J, Schauerte C, Zuber J et al (2015) Hyperactivation of mTORC1 and mTORC2 by multiple oncogenic events causes addiction to eIF4E-dependent mRNA translation in T-cell leukemia. Oncogene 34(27):3593–3604
Sato A, Ueno H, Takase A, Ando A, Sekine Y, Yano T (2016) Cytotoxicity induced by a redox-silent analog of Tocotrienol in human mesothelioma H2452 cell line via suppression of cap-dependent protein translation. Anticancer Res 36(4):1527–1533
Whitson BA, Kratzke RA (2006) Molecular pathways in malignant pleural mesothelioma. Cancer Lett 239(2):183–189
Lee AY, Raz DJ, He B, Jablons DM (2007) Update on the molecular biology of malignant mesothelioma. Cancer 109(8):1454–1461
Carbone M, Rizzo P, Grimley PM, Procopio A, Mew DJY, Shridhar V, De Batolomeis A, Esposito V, Giuliano MT, Steinberg SM, Levine AS, Giordano A, Pass HI (1997) Simian virus-40 large-T antigen binds p53 in human mesotheliomas. Nat Med 3(8):908–912
Carbone M, Kratzke RA, Testa JR (2002) The pathogenesis of mesothelioma. Semin Oncol 29(1):2–17
Antman KH, Pass HI, Recht A (1989) Benign and malignant mesothelioma. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer: principle and practices of oncology, 3rd edn. J.B.Lippincot, Philadelphia, pp 1399–1417
Jacobson BA, De A, Kratzke MG, Patel MR, Dixon JJ, Whitson BA, Sadiq AA, Bitterman PB, Polunovsky VA, Kratzke RA (2009) Activated 4E-BP1 represses tumourigenesis and IGF-I-mediated activation of the eIF4F complex in mesothelioma. Br J Cancer 101(3):424–431
Li S, Takasu T, Perlman DM, Peterson MS, Burrichter D, Avdulov S et al (2003) Translation factor eIF4E rescues cells from Myc-dependent apoptosis by inhibiting cytochrome c release. J Biol Chem 278(5):3015–3022
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5(10):R80
Wilson CL, Miller CJ (2005) Simpleaffy: a BioConductor package for Affymetrix quality control and data analysis. Bioinformatics 21(18):3683–3685
Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG et al (2005) Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 33(20):e175
Sandberg R, Larsson O (2007) Improved precision and accuracy for microarrays using updated probe set definitions. BMC Bioinformatics 8:48
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98(9):5116–5121
Larsson O, Li S, Issaenko OA, Avdulov S, Peterson M, Smith K et al (2007) Eukaryotic translation initiation factor 4E induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res 67(14):6814–6824
Wennmalm K, Wahlestedt C, Larsson O (2005) The expression signature of in vitro senescence resembles mouse but not human aging. Genome Biol 6(13):R109
Li S, Perlman DM, Peterson MS, Burrichter D, Avdulov S, Polunovsky VA et al (2004) Translation initiation factor 4E blocks endoplasmic reticulum-mediated apoptosis. J Biol Chem 279(20):21312–21317
Larsson O, Perlman DM, Fan D, Reilly CS, Peterson M, Dahlgren C et al (2006) Apoptosis resistance downstream of eIF4E: posttranscriptional activation of an anti-apoptotic transcript carrying a consensus hairpin structure. Nucleic Acids Res
Greillier L, Baas P, Welch JJ, Hasan B, Passioukov A (2008) Biomarkers for malignant pleural mesothelioma: current status. Mol Diagn Ther 12(6):375–390
Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM et al (2004) Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell 5(6):553–563
Sekiyama N, Arthanari H, Papadopoulos E, Rodriguez-Mias RA, Wagner G, Leger-Abraham M (2015) Molecular mechanism of the dual activity of 4EGI-1: dissociating eIF4G from eIF4E but stabilizing the binding of unphosphorylated 4E-BP1. Proc Natl Acad Sci U S A 112(30):E4036–E4045
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21(14):2636–2644
Kindler HL, van Meerbeeck JP (2002) The role of gemcitabine in the treatment of malignant mesothelioma. Semin Oncol 29(1):70–76
Fukazawa T, Matsuoka J, Naomoto Y, Maeda Y, Durbin ML, Tanaka N (2008) Malignant pleural mesothelioma-targeted CREBBP/EP300 inhibitory protein 1 promoter system for gene therapy and virotherapy. Cancer Res 68(17):7120–7129
Zhang C, Li K, Wei L, Li Z, Yu P, Teng L et al (2007) p300 expression repression by hypermethylation associated with tumour invasion and metastasis in oesophageal squamous cell carcinoma. J Clin Pathol 60(11):1249–1253
Miura TA, Cook JL, Potter TA, Ryan S, Routes JM (2007) The interaction of adenovirus E1A with p300 family members modulates cellular gene expression to reduce tumorigenicity. J Cell Biochem 100(4):929–940
Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J et al (2009) Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A 106(32):13481–13486
Okino ST, Pookot D, Basak S, Dahiya R (2009) Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res (Phila) 2(3):251–256
Ito T, Tsukumo S, Suzuki N, Motohashi H, Yamamoto M, Fujii-Kuriyama Y et al (2004) A constitutively active arylhydrocarbon receptor induces growth inhibition of jurkat T cells through changes in the expression of genes related to apoptosis and cell cycle arrest. J Biol Chem 279(24):25204–25210
Liang Y, Lin SY, Brunicardi FC, Goss J, Li K (2009) DNA damage response pathways in tumor suppression and cancer treatment. World J Surg 33(4):661–666
Ouyang G, Yao L, Ruan K, Song G, Mao Y, Bao S (2009) Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int
Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL (2009) Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys 486(2):95–102
He H, Dai F, Yu L, She X, Zhao Y, Jiang J et al (2002) Identification and characterization of nine novel human small GTPases showing variable expressions in liver cancer tissues. Gene Expr 10(5–6):231–242
Bani MR, Nicoletti MI, Alkharouf NW, Ghilardi C, Petersen D, Erba E et al (2004) Gene expression correlating with response to paclitaxel in ovarian carcinoma xenografts. Mol Cancer Ther 3(2):111–121
Asada K, Miyamoto K, Fukutomi T, Tsuda H, Yagi Y, Wakazono K et al (2003) Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology 64(4):380–388
Li DQ, Wang L, Fei F, Hou YF, Luo JM, Zeng R et al (2006) Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics 6(11):3352–3368
van't Veer MB, Brooijmans AM, Langerak AW, Verhaaf B, Goudswaard CS, Graveland WJ et al (2006) The predictive value of lipoprotein lipase for survival in chronic lymphocytic leukemia. Haematologica 91(1):56–63
Polunovsky VA, Bitterman PB (2006) The cap-dependent translation apparatus integrates and amplifies cancer pathways. RNA Biol 3(1):10–17
Bitterman PB, Polunovsky VA (2012) Attacking a nexus of the oncogenic circuitry by reversing aberrant eIF4F-mediated translation. Mol Cancer Ther 11(5):1051–1061
Jacobson BA, Thumma SC, Jay-Dixon J, Patel MR, Dubear Kroening K, Kratzke MG et al (2013) Targeting eukaryotic translation in mesothelioma cells with an eIF4E-specific antisense oligonucleotide. PLoS One 8(11):e81669
Jacobson BA, Alter MD, Kratzke MG, Frizelle SP, Zhang Y, Peterson MS et al (2006) Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res 66(8):4256–4262
von der Haar T, Gross JD, Wagner G, McCarthy JE (2004) The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol 11(6):503–511
Watkins SJ, Norbury CJ (2002) Translation initiation and its deregulation during tumorigenesis. Br J Cancer 86(7):1023–1027
Acknowledgements
This work is supported by a generous grant from the Mesothelioma Applied Research Foundation and is dedicated to the memory of Christopher Stoeckler. The authors are grateful to Dr. Joshua Baller, Research Informatics Scientific and Operations Lead with the Minnesota supercomputing Institute, for his bioinformatics assistance. We thank Michael J. Franklin, MS for editing the manuscript. All authors have read the journal’s authorship agreement and the policy on potential conflicts of interest.
Funding
This work is supported by a generous grant from the Mesothelioma Applied Research Foundation. Grant identification is: “Targeting cap-mediated translation for mesothelioma therapy”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no potential conflicts of interest.
Ethical approval
This article does not contain studies involving human participants or animals.
Electronic supplementary material
Supplemental Table 1
(XLS 439 kb)
Rights and permissions
About this article
Cite this article
De, A., Jacobson, B.A., Peterson, M.S. et al. 4EGI-1 represses cap-dependent translation and regulates genome-wide translation in malignant pleural mesothelioma. Invest New Drugs 36, 217–229 (2018). https://doi.org/10.1007/s10637-017-0535-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0535-z