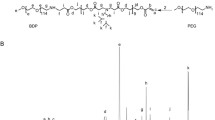
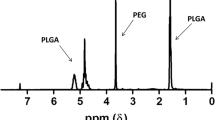
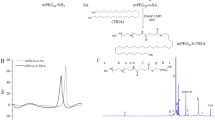
Summary
Purpose Raloxifene (RA) receptors have over-expressed GPER-positive breast cancer tumors. The purpose of this work was to evaluate the antitumor activity and pharmacokinetic behavior of docetaxel (DTX), loaded in RA-targeted nanomicelles, which were designed to overcome a lack of specific distribution and inadequate DTX concentration in tumor tissues, as well as its cytotoxicity and damage to normal tissues. Methods DTX-loaded RA-targeted poly(styrene maleic acid) (SMA)- poly(amide-ether-esterimide)-poly(ethylene glycol) (PAEEI-PEG) nanomicelles were prepared; then, their antitumor activity and survival rate were studied in MC4-L2 tumors induced in BALB/c mice. The pharmacokinetics of DTX-loaded SMA-PAEEI-PEG-RA micelles was also investigated in comparison with free DTX. Results DTX-loaded SMA-PAEEI-PEG-RA micelles inhibited tumor growth considerably and increased animal survival as compared to free DTX and non-targeted micelles. SMA-PAEEIPEG-RA micelles enhanced significantly the area under the curve (AUC0-∞) 1.3 times as compared to free DTX and reduced clearance (CL) from 410.43 ml/kg.h (for free DTX) to 308.8 ml/kg.h (for SMA-PAEEI-PEG-RA micelles). Volume of distribution (Vdss) was also reduced 1.4 times as compared to free DTX. RA-targeted micelles increased tumor inhibition rate (TIR) 1.3 times and median survival time (MST) >1.5 times compared to free DTX. Percentage increase in life span (%ILS) was also enhanced significantly from 41.66% to >83.33% in MC4-L2 tumor-bearing BALB/c mice. Discussion All studies in this work showed the potential of DTX-loaded SMA-PAEEI-PEG-RA micelles in the treatment of GPER-positive receptor breast cancer tumors.






Similar content being viewed by others
References
Jain V, Jain S, Mahajan S (2014) Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr Drug Deliv 12(2):177–191
Liang X-J, Chen C, Zhao Y, Wang PC (2010) Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. Methods Mol Biol 596:467–488
Byrne J, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60(15):1615–1626
Minko T, Rodriguez-Rodriguez L, Pozharov V (2013) Nanotechnology approaches for personalized treatment of multidrug resistant cancers. Adv Drug Deliv Rev 65(13):1880–1895
Gemignani ML, Armstrong DK (2014) Breast cancer. Gynecol Oncol 132(2):264–267
Liu B, Yang M, Li R, Ding Y, Qian X, Yu L, Jiang X (2008) The antitumor effect of novel docetaxel-loaded thermosensitive micelles. Eur J Pharm Biopharm 69(2):527–534
Pazdur R, Cortes JE (1995) Docetaxel. J Clin Oncol 13(10):2643–2655
Bharali DJ, Khalil M, Gurbuz M, Simone TM, Mousa SA (2009) Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int J Nanomedicine 4(1):1–7
Liu J, Zeng F, Allen C (2007) In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur J Pharm Biopharm 65(3):309–319
Qu G, Yao Z, Zhang C, Wu X, Ping Q (2009) PEG conjugated N-octyl-O-sulfate chitosan micelles for delivery of paclitaxel: in vitro characterization and in vivo evaluation. Eur J Pharm Sci 37(2):98–105
Alexis F, Pridgen E, Molnar LK, Farokhzad OC (2000) Factors affecting the clearance and biodistribution of polymeric nanoparticles. Biotechnology 18:412–420
Manchun S, Dass CR, Sriamornsak P (2012) Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci 90(11):381–387
Ganta S, Devalapally H, Shahiwala A, Amiji M (2008) A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 126(3):187–204
Yang C, Ebrahim Attia AB, Tan JPK, Ke X, Gao S, Hedrick JL, Yang Y-Y (2012) The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials 33(10):2971–2979
Lappano R, Pisano A, Maggiolini M (2014) GPER function in breast cancer: an overview. Front Endocrinol 5:66
Cheng S-B, Graeber CT, Quinn JA, Filardo EJ (2011) Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids 76(9):892–896
Thomas P, Pang Y, Filardo E, Dong J (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146(2):624–632
Prossnitz ER, Sklar LA, Oprea TI, Arterburn JB (2008) GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol Sci 29(3):116–123
Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ (1997) Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 45(3):607–617
Hol T, Cox MB, Bryant HU, Draper MW (1997) Selective estrogen receptor modulators and postmenopausal women's health. J Women's Health 6(5):523–531
Sporn MB, Dowsett SA, Mershon J, Bryant HU (2004) Role of raloxifene in breast cancer prevention in postmenopausal women: Clinical evidence and potential mechanisms of action. Clin Ther 26(6):830–840
Varshosaz J, Enteshari S, Hassanzadeh F, Hashemi Bani B (2017) Synthesis of novel polystyrene-poly(amide-ether-ester-imide)-poly(ethylene glycol) co-polymeric micelles loaded with docetaxel: Physicochemical evaluation and cytotoxic effects on breast cancer cell lines. Mater Sci Mater Med (in press)
Varshosaz J, Enteshari S, Hassanzadeh F, Hashemi Bani B (2017) Raloxifene targeted styrene maleic acid (SMA)-poly ether ester imide–poly ethylene glycol (PAEEI-PEG) copolymeric micelles for the targeted delivery of docetaxel: Physicochemical evaluation and cytotoxic effects on breast cancer cell lines. Polos One (in press)
Lanari C, Lüthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, Sanjuan N, Merani S, Molinolo AA (2001) Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro effects of estrogens and progestins. Cancer Res 61(1):293–302
Taymouri S, Varshosaz J, Hassanzadeh F, Javanmard SH, Mahzouni P (2016) Pharmacokinetics, organ toxicity and antitumor activity of docetaxel loaded in folate targeted cholesterol based micelles. Curr Drug Deliv 13(4):545–556
Taheri A, Dinarvand R, Nouri FS, Khorramizadeh MR, Borougeni AT, Mansoori P, Atyabi F (2011) Use of biotin targeted methotrexate-human serum albumin conjugated nanoparticles to enhance methotrexate antitumor efficacy. Int J Nanomedicine 6:1863–1874
Zhao M, Su M, Lin X, Luo Y, He H, Cai C, Tang X (2010) Evaluation of docetaxel-loaded intravenous lipid emulsion: pharmacokinetics, tissue distribution, antitumor activity, safety and toxicity. Pharm Res 27(8):1687–1702
Ernsting MJ, Tang W-L, MacCallum NW, Li S-D (2012) Preclinical pharmacokinetic, biodistribution, and anti-cancer efficacy studies of a docetaxel-carboxymethylcellulose nanoparticle in mouse models. Biomaterials 33(5):1445–1454
Wang X, Li J, Wang Y, Cho KJ, Kim G, Gjyrezi A, Koenig L, Giannakakou P, Shin HJC, Tighiouart M (2009) HFT-T, a targeting nanoparticle, enhances specific delivery of paclitaxel to folate receptor-positive tumors. ACS Nano 3(10):3165–3174
Deng C, Jiang Y, Cheng R, Meng F, Zhong Z (2012) Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects. Nano Today 7(5):467–480
Zhao L, Y-m W, X-d Z, Liang Y, X-m Z, Li W, B-b L, Wang Y, Yu Y (2009) PK and tissue distribution of docetaxel in rabbits after i.v. administration of liposomal and injectable formulations. J Pharm Biomed Anal 49(4):989–996
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63(3):136–151
Rezazadeh M, Emami J, Hasanzadeh F, Sadeghi H, Minaiyan M, Mostafavi A, Rostami M, Lavasanifar A (2016) In vivo pharmacokinetics, biodistribution and anti-tumor effect of paclitaxel-loaded targeted chitosan-based polymeric micelle. Drug Deliv 23(5):1707–1717
Hu F-Q, Meng P, Dai Y-Q, Du Y-Z, You J, Wei X-H, Yuan H (2008) PEGylated chitosan-based polymer micelle as an intracellular delivery carrier for anti-tumor targeting therapy. Eur J Pharm Biopharm 70(3):749–757
Sausville EA, Burger AM (2006) Contributions of human tumor xenografts to anticancer drug development. Cancer Res 66(7):3351–3354
Song Y, Tian Q, Huang Z, Fan D, She Z, Liu X, Cheng X, Yu B, Deng Y (2014) Self-assembled micelles of novel amphiphilic copolymer cholesterol-coupled F68 containing cabazitaxel as a drug delivery system. Int J Nanomedicine 9:2307
Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R (2006) Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A 103(16):6315–6320
Acknowledgments
Financial support of the project by the Research Vice Chancellery of Isfahan University of Medical Sciences is appreciated. The authors gratefully appreciate the technical assistance of Dr. Mohajeri and MS Mahmoodi in doing immunohistochemical tests.
Funding
This study was funded by grant number 394283 of Isfahan University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Saeede Enteshari declares that she has no conflict of interest. Jaleh Varshosaz declares that she has no conflict of interest. Mohsen Minayian declares that he has no conflict of interest. Farshid Hassanzadeh declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Enteshari, S., Varshosaz, J., Minayian, M. et al. Antitumor activity of raloxifene-targeted poly(styrene maleic acid)-poly (amide-ether-ester-imide) co-polymeric nanomicelles loaded with docetaxel in breast cancer-bearing mice. Invest New Drugs 36, 206–216 (2018). https://doi.org/10.1007/s10637-017-0533-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0533-1