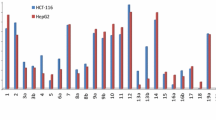
A heterocyclization reaction of 5,6-diamino-1-methyluracil and 2-oxopentanedioic acid was used in this study to synthesize 3-(1-methyl-2,4,7-trioxo-1,2,3,4,7,8-hexahydropteridin-6-yl)propanoic acid, intramolecular cyclization of which led to the formation of a tricyclic lactone, namely, 1-methyl-6,7-dihydro-2H-pyrano[3,2-g]pteridine-2,4,8-(1H,3H)-trione. Reactions of the latter with N-nucleophiles gave a series of amides – structural analogs of antifolates. The structure and identity of the synthesized compounds were confirmed by IR spectroscopy, 1H and 13C NMR spectroscopy, LC-MS analysis, and mass spectrometry. It was established that the synthesized compounds showed cytotoxic effects against human hepatocellular carcinoma (HepG2) cells and may be of interest for further studies of their antitumor activity against other cell lines.

Similar content being viewed by others
References
Qujeq, D.; Ahmadi, H. Am. J. Nephrol. 2001, 21, 340.
Cunnington, C.; Van Assche, T.; Shirodaria, C.; Kylintireas, I.; Lindsay, A. C.; Lee, J. M.; Antoniades, C.; Margaritis, M.; Lee, R.; Cerrato, R.; Crabtree, M. J.; Francis, J. M.; Sayeed, R.; Ratnatunga, C.; Pillai, R.; Choudhury, R. P.; Neubauer, S.; Channon, K. M. Circulation 2012, 125, 1356.
Werner-Felmayer, G.; Golderer, G.; Werner, E. R. Curr. Drug Metab. 2002, 3, 159.
Shalini, A.; Pavitra, K. K. Agro Food Ind. Hi-Tech 2012, 23, 25.
Kompis, I. M.; Islam, K.; Then, R. L. Chem. Rev. 2005, 105, 593.
Palanki, M. S. S.; Dneprovskaia, E.; Doukas, J.; Fine, R. M.; Hood, J.; Kang, X.; Lohse, D.; Martin, M.; Noronha, G.; Soll, R. M.; Wrasidlo, W.; Yee, S.; Zhu, H. J. Med. Chem. 2007, 50, 4279.
Kuroda, Y.; Isarai, K.; Murata, S. Heterocycl. Commun. 2012, 18, 117.
Murata, S.; Ichinose, H.; Urano, F. In Bioactive Heterocycles II. Topics in Heterocyclic Chemistry; Eguchi, S., Ed.; Springer: Berlin, Heidelberg, 2007, Vol. 8, p. 127.
Kim, Y.; Kang, Y.; Baek, D. Bull. Korean Chem. Soc. 2001, 22, 141.
Guiney, D.; Gibson, C. L.; Suckling, C. J. Org. Biomol. Chem. 2003, 1, 664.
Kazunin, M. S.; Voskoboynik, O. Yu.; Nosulenko, I. S.; Berest, G. G.; Sergeieva, T.; Okovytyy, S.; Karpenko, O. V.; Priimenko, B. O.; Kovalenko, S. I. J. Heterocycl. Chem. 2018, 55, 1033.
Pfleiderer, W. Angew. Chem. 1963, 75, 993.
Valeur, E.; Bradley, M. Chem. Soc. Rev. 2009, 38, 606.
El-Sabbagh, O. I.; El-Sadek, M. E.; El-Kalyoubi, S.; Ismail, I. Arch. Pharm. Chem. Life Sci. 2007, 340, 26.
Jacobsen, N. E. NMR Spectroscopy Explained: Simplified Theory, Applications and Examples for Organic Chemistry and Structural Biology; John Wiley & Sons, Inc., 2007.
Mass Spectrometry in Organic Chemistry [in Russian]; Lebedev, А. Т.; Ed.; BINOM. Laboratoriya Znanii: Moscow, 2003.
Daele, V. J.; Blancquaert, D.; Kiekens, F.; Van Der Straeten, D.; Lambert, W. E.; Stove, C. P. Food Chem. 2016, 194, 1189.
Mitroshina, E. V.; Mishchenko, Т. А.; Vedunova, M. V. Determination of Cell Culture Viability: a Study Aid [in Russian]; Nizhegorod Lobachevsky State University: Nizhny Novgorod, 2015. http://www.neuro.unn.ru/sites/default/files/opredelenie_zhiznesposobnosti.pdf
Mitskevich, P. B.; Kosmacheva, S. M.; Ibragimova, Zh. A.; Shman, T. V.; Mislitskii, V. F. Application of МТТ test for Evaluating the Sensitivity of Leukemia Cells to Cytostatic Drugs in vitro and Predicting the Response of Leukemia to Chemotherapy; User Manual [in Russian]; Ministry of Health of the Republic of Belarus: Minsk. Registration number 11111102, approval date February 13, 2003. http://med.by/methods/pdf/111-1102.pdf
Mosmann, T. J. Immunol. Methods 1983, 65, 55.
Cancer Cell Culture: Methods and Protocols (Methods in Molecular Medicine); Langdon, S. P., Eds.; Humana Press: Totowa, 2004, p. 165.
Supino, R. In In Vitro Toxicity Testing Protocols (Methods in Molecular Biology); O'Hare, S.; Atterwill, C. K., Eds.; Springer: New York, 1995, Vol. 43, p. 138.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(4/5), 408–415
Rights and permissions
About this article
Cite this article
Kazunin, M.S., Voskoboynik, O.Y., Shatalova, O.M. et al. Amides of substituted 3-(pteridin-6-yl)propanoic acids: synthesis, spectral characteristics, and cytotoxic activity. Chem Heterocycl Comp 55, 408–415 (2019). https://doi.org/10.1007/s10593-019-02473-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02473-x