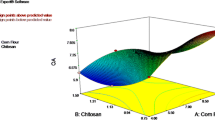
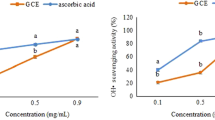
Abstract
The present study investigated chitosan nanoparticle (CS-TPP NPs) preparation by ionic gelation process with chitosan (CS) and tripolyphosphate (TPP). The structural characterization of nanoparticles was studied using Dynamic Light Scattering, Nuclear Magnetic Resonance, Fourier Transform Infrared (FTIR) and X-Ray Diffraction spectroscopies. FTIR spectra confirmed that the tripolyphosphoric groups of TPP linked with ammonium groups of CS in the prepared nanoparticles. CS-TPP NPs exhibited higher antioxidant activity and an interesting antimicrobial potential. In fact, there was no report available in literature on using CS nanoparticles for the surimi preservation. Further, data revealed that nanoparticle incorporation in fish surimi effectively inhibited thiobarbituric acid substances and conjugated dienes formation, and thereby, retarding lipid oxidation. Results showed that CS-TPP NPs were able to hinder fish surimi myoglobin oxidation with a significant improvement in the transformation of Heme iron. In addition, CS nanoparticles exhibited a distinguishable antimicrobial activity in the stored surimi during cold storage of 9 days. Thus, nanoparticles could be used as a natural ingredient to prevent lipid oxidation in surimi based food systems for the development of novel healthy fish products and addresses consumer demands for functional fish products.






Similar content being viewed by others
References
Akmaz S, AdJgüzel ED, Yasar M, Erguven O (2013) The effect of Ag content of the chitosan-silver nanoparticle nomposite material on the structure and antibacterial activity. Adv Mater Sci Eng. Article ID 690918, 6
Ali WS, Joshi M, Rajendran S (2010) Modulation of size, shape and surface charge of chitosan nanoparticles with reference to antimicrobial activity. Adv Sci Lett 3:452–460
Alishahi A, Mirvaghefi A, Tehrani MR, Farahmand H, Shojaosadati SA, Dorkoosh FA, Elsabee MZ (2011) Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chem 126:935–940
Anraku M, Fujii T, Furutani N, Kadowaki D, Maruyama T, Otagiri M, Gebicki JM, Tomida H (2009) Antioxidant effects of a dietary supplement: reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem Toxicol 47:104–109
Arulmozhi V, Pandian K, Mirunalini S (2013) Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf B 110:313–320
Barbi MS, Carvalho FC, Kiill CP, Barud HS, Santagneli SH, Ribeiro SJL, Gremião MPD (2015) Preparation and characterization of chitosan nanoparticles for zidovudine nasal delivery. Nanosci Nanotechnol 15:865–874
Benjakul S, Bauer F (2001) Biochemical and physicochemical changes in catfish muscle as influenced by different freeze-thaw cycles. Food Chem 72:207–217
Bertrand N, Wu J, Xu X, Kamaly N, Farokhad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25
Calero N, Munoz J, Ramirez P, Guerrero A (2010) Flow behaviour, linear viscoelasticity and surface properties of chitosan aqueous solutions. Food Hydrocoll 24:659–666
Calvo P, Remunan Lopez C, Vila Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2004) Characteristics and gel properties of muscles from sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) caught in Thailand. Food Res Int 37:1021–1030
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2005) Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem 93:607–617
Chaijan M, Benjakul S, Visessanguan W, Faustman C (2006) Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chem 99:83–91
Chaijan M, Panpipat W, Benjakul S (2010) Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chem 121:85–92
Chen HH (2002) Decoloration and gel-forming ability of horse mackerel mince by air-flotation washing. J Food Sci 67:2970–2975
Chi CF, Hu FY, Wang B, Ren XJ, Deng SG, Wu CW (2015) Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem 168:662–667
Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S (2011) Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol 18:591–599
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D’Alessio M, Zambonin PG, Traversa E (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 17:5255–5262
Coelho G, Weschenfelder Â, Meinert E, Amboni R, Beirão LH (2007) Effects of starch properties on textural characteristics of fish burgers: sensory and instrumental approaches. Boletim Centro de Pesquisa de Processamento de Alimentos 25:37–50
Colmenero FJ, Barreto G, Fernández P, Carballo J (1996) Frozen storage of bologna sausages as a function of fat content and of levels of added starch and egg white. Meat Sci 42:325–332
Darmadji P, Izumimoto M (1994) Effect of chitosan in meat preservation. Meat Sci 38:243–254
de Pinho Neves AL, Milioli CC, Müller L, Riella HG, Kuhnen NC, Stulzer HK (2014) Factorial design as tool in chitosan nanoparticles development by ionic gelation technique. Colloids Surf A Physicochem Eng Asp 445:34–39
Dey SS, Dora KC, Raychaudhuri U, Ganguly S (2013) Cryoprotective effect of shrimp waste protein hydrolysate on croaker surimi protein and gel characteristics during frozen storage. Fish Technol 50:50–59
Divya K, Jisha MS (2017) Chitosan nanoparticles preparation and applications. Environ Chem Lett 16:101–112
Divya K, Vijayan S, Jisha MS (2018) Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int J Biol Macromol 114:572–577
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym 75:385–389
Dudhani AR, Kosaraju SL (2010) Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym 81:243–251
Esterbauer H, Cheeseman KH, Dianzani MU, Poli G, Slater TI (1982) Separation andcharacterization of the aldehydic products of lipid peroxidation stimulated by ADP Fe2+in rat liver microsomes. Biochem J 208:129–140
Fernandez-Kim SO (2004) Physicochemical and functional properties of crawfish chitosan as affected by different processing protocols. Thesis
Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA (2007) Effect of rosemary extract, chitosan and α-tocopherol onmicrobiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C. Meat Sci 76:172–181
Gilbert RJ, de Louvois J, Donovan T, Little C, Nye K, Ribeiro CD, Richards J, Roberts D, Bolton FJ (2000) Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale. PHLS advisory committee for food and dairy products. Commun Dis Public Health 3:163–167
Gomez-Basauri JV, Regenstein JM (1992) Vacuum packaging, ascorbic acid and frozen storage effects on Heme and Nonheme iron content of Mackerel. J Food Sci 57:1337–1339
Gu HW, Ho PL, Tong E, Wang L, Xu B (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 3:1261–1263
Haard NF (1992) Biochemistry and chemistry of color and color change in seafoods. Advances in Seafood Biochemistry. Technomic Publishing Co, Inc, Lancester, Penn Sylvania, pp 312–319
Hajji S, Younes I, Ghorbel-Bellaaj O, Hajji R, Rinaudo M, Nasri M, Jellouli K (2014) Structural differences between chitin and chitosan extracted from three different marine sources. Int J Biol Macromol 65:298–306
Hamdi M, Hajji S, Affes S, Taktak W, Maâlej H, Nasri M, Nasri R (2017) Development of a controlled bioconversion process for the recovery of chitosan from blue crab (Portunus segnis) exoskeleton. Food Hydrocoll 101:455–463
Hansen LJ, Sereika HE (1969) Factors affecting color stability of prepackaged frozen fresh beef in display cases. Illum Eng 64:620–624
Hebeish A, Sharaf S, Farouk A (2013) Utilization of chitosan nanoparticles as a green finish in multifunctionalization of cotton textile. Int J Biol Macromol 60:10–17
Horita CN, Morgano MA, Celeghini RMS, Pollonio MAR (2011) Physicochemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Sci 89:426–433
Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F (2015) Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll 44:172–182
Hosseini SF, Soleimani MR, Nikkhah M (2018) Chitosan/sodium tripolyphosphate nanoparticles as efficient vehicles for antioxidant peptidic fraction from common kilka. Int J Biol Macromol 111:730–737
Huang ZM, Zhang Y, Kotaki M, Ramakrishnaj S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253
Jingou J, Shilei H, Weiqi L, Danjun W, Tengfei W, Yi X (2011) Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded chitosan/cyclodextrin nanoparticles and in vitro release study. Colloids Surf B 83:103–107
Jung KH, Lee JH, Park JW, Quach HT, Moon SH, Cho YS, Lee KH (2015) Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int J Pharm 478:251–257
Kafshgari MH, Khorram M, Mansouri M, Samimi A, Osfouri S (2012) Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym J 21:99–107
Kanatt SR, Chander R, Sharma A (2008) Chitosan and mint mixture: a new preservative for meat and meat products. Food Chem 107:845–852
Kaya M, Mujtaba M, Ehrlich H, Salaberria AM, Baran T, Amemiya CT, Galli R, Akyuz L, Sargin I, Labidi J (2017) On chemistry of γ-chitin. Carbohydr Polym 176:177–186
Ke PJ, Cervantes E, Robles-Martinez C (1984) Determination of thiobarbituric acid reactive substances (TBARS) in fish tissue by an improved distillation spectrophotometric method. J Sci Food Agric 35:1248–1254
Kim DG, Jeong YI, Choi C, Roh SH, Kang SK, Jang MK, Nah JW (2006) Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int J Pharm 319:130–138
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1–21
Kim JH, Kim SI, Kwon IB, Kim MH, Lim JI (2013) Simple fabrication of silver hybridized porous chitosan-based patch for transdermal drug-delivery system. Mater Lett 95:48–51
Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Lee S, Joo ST, Alderton AL, Hill DW, Faustman C (2003) Oxymyoglobin and lipid oxidation in yellow fin tuna (Thunnus albacares) loins. J Food Sci 68:1664–1668
León FJC, Lizardi-Mendoza J, Arguelles-Monal W, Carvajal-Millan E, Lopez Franco YL, Goycoolea FM (2017) Supercritical CO2 dried chitosan nanoparticles: production and characterization. RSC Adv 7:30879–30885
Li Y, Song H, Xiong S, Tian T, Liu T, Sun Y (2005a) Chitosan-stablized bovine serum albumin nanoparticles having ability to control the release of NELL-1 protein. Int J Biol Macromol 109:672–680
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M (2005b) Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials 26:3919–3928
Maciel VBV, Yoshida CMP, Pereira SMSS, Goycoolea FM, Franco TT (2017) Electrostatic self-assembled chitosan-pectin nano- and microparticles for insulin delivery. Molecules 22:1707
Mao L, Wu T (2007) Gelling properties and lipid oxidation of kamaboko gels from grass carp (Ctenopharyngodon idellus) influenced by chitosan. J Food Eng 82:128–134
Meshkini S, Tafy AA, Tukmechi A, Farhang-Pajuh F (2012) Effects of chitosan on hematological parameters and stress resistance in rainbow trout (Oncorhynchus mykiss). Vet Res Forum 3:49–54
Nallamuthu I, Devi A, Khanum F (2015) Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J Pharm Sci 10:203–211
Nguyen TV, Nguyen TTH, Wang SL, Khanh Vo TP, Nguyen AD (2017) Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res Chem Intermed 43:3527–3537
Nishimura T (2010) The role of intramuscular connective tissue in meat texture. Animal Sci J 81:21–27
Ochiai Y, Ochiai L, Hashimoto K, Watabe S (2001) Quantitative estimation of dark muscle content in the mackerel meat paste and its products using antisera against myosin light chains. J Food Sci 66:1301–1305
Ocloo FCK, Quayson ET, Adu-Gyamfi A, Quarcoo EA, Asare D, Serfor Armah Y, Woode BK (2011) Physicochemical and functional characteristics of radiation-processed shrimp chitosan. J Radiat Phys Chem 80:837–841
Phromsopha T, Baimark Y (2010) Chitosan microparticles prepared by the water-in-oil emulsion solvent diffusion method for drug delivery. Biotechnology 9:61–66
Piyakulawat P, Praphairaksit N, Chantarasiri N, Muangsin N (2007) Preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS Pharm Sci Technol 8:120–130
Plainsirichai M, Leelaphatthanapanich S, Wongsachai N (2014) Effect of chitosan on the quality of rose apples (Syzygium Agueum Alston) cv. Tabtim Chan stored at an ambient temperature. APCBEE Proc 8:317–322
Postnikova GB, Tselikova SV, Kolaeva SG, Solomonov NG (1999) Myoglobin content in skeletal muscles of hibernating ground squirrels rises in autumn and winter. Comp Biochem Physiol Part B 124:35–37
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339:2693–2700
Rajalekshmi M, Mathew PT (2007) Colour and textural parameters of threadfin bream surimi during frozen storage as affected by cryoprotectants and chitosan. Fish Technol 44:55–64
Rodrigues S, Costa AM, Grenha A (2012) Chitosan/carrageenan nanoparticles: effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr Polym 89:282–289
Rokhade AP, Agnihotri SA, Patil SA, Mallikarjuna NN, Kulkarni PV, Aminabhavi TM (2006) Semi-interpen etrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr Polym 65:243–252
Ruan S, Yuan M, Zhang L, Hu G, Chen J, Cun X, Gao H (2015) Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 37:425–435
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10:37–51
Tokumitsu H, Ichikawa H, Fukumori Y (1999) Chitosan-gadopentetic acid complex. Nanoparticles for gadolinium neutron-capture therapy of cancer: preparation by novel emulsion-droplet coalescence technique and characterization. Pharm Res 16:1830–1835
Vasconcelos F, Cristol S, Paul JF, Tricot GG, Amoureux JP, Montagne L, Mauri F, Delevoye L (2008) 17O solid-state NMR and first-principles calculations of sodium trimetaphosphate (Na3P3O9), tripolyphosphate (Na5P3O10) and pyrophosphate (Na4P2O7). Inorg Chem 47:7327–7337
Viji P, Tanuja S, Ninan G, Zynudheen AA, Lalitha KV (2014) Quality characteristics and shelf life of sutchi cat fish (Pangasianodon hypophthalmus) steaks during refrigerated storage. Int J Agric Food Sci Technol 5:105–116
Wang YF, Huang SR, Shao SH, Qian L, Xu P (2012) Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind Crops Prod 37:520–526
Yang HC, Wang WH, Huang KS, Hon MH (2010) Preparation and application of nanochitosan to finishing treatment with anti-microbial and anti-shrinking properties. Carbohydr Polym 79:176–179
Yen GC, Wu J (1999) Antioxidant and radical scavenging properties of extracts from Ganoderma tsugae. Food Chem 65:375–379
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49:4083–4089
Acknowledgments
This work was supported by Grants from Ministry of Higher Education and Scientific Research of Tunisia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajji, S., Hamdi, M., Boufi, S. et al. Suitability of chitosan nanoparticles as cryoprotectant on shelf life of restructured fish surimi during chilled storage. Cellulose 26, 6825–6847 (2019). https://doi.org/10.1007/s10570-019-02555-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02555-1