Abstract
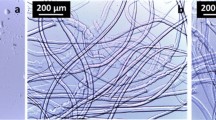
The production of cellulosic man made fibres by the viscose process has been known for more than 120 years now, but still some aspects are not sufficiently understood in detail. The carbohydrates in the pulp are exposed to varying conditions during the manufacturing process. In the first production step of steeping, the strong alkaline treatment leads to undesirable loss reactions of the cellulose. In this study, a comprehensive kinetic model was developed for process simulation of cellulose degradation for the fist time comprising primary and secondary peeling, stopping and alkaline hydrolysis. A total chlorine free bleached beech sulfite pulp was treated with 18 % sodium hydroxide at 40, 50 and 60 °C for time periods up to 80 h. The corresponding reaction rates, activation energies and frequency factors for all reaction steps were calculated. The peeling-off reaction was of great significance for the cellulose yield loss, due to a contribution of the secondary peeling after random chain scission. The moderate decrease of the intrinsic viscosity and the changes in molar mass distribution indicated the validity of the assumption. Further, a reduction of the carbonyl and an increase of the carboxyl groups in the cellulose were observed due to the formation of the stable metasaccharinic acid at the reducing ends of the molecules. The fibre morphology was investigated by SEM measurements. Already short alkaline treatment times favored the dissolution of fibril fragments from the fibre surface leading to a smooth fibre surface.








Similar content being viewed by others
Abbreviations
- BAR:
-
Benzilic acid rearrangement
- CCOA:
-
Carbazole-9-carbonyloxyamine
- D :
-
Overall carbohydrate yield loss
- DMAc:
-
N,N-Dimethylacetamide
- DP:
-
Degree of polymerisation
- FDAM:
-
9H-fluoren-2-yl-diazomethane
- FE-SEM:
-
Scanning electron microscope
- H :
-
Material degraded by hydrolysis
- HPLC:
-
High performance liquid chromatography
- ISA:
-
Isosaccharinic acid
- k h :
-
Rate constant of the alkaline hydrolysis
- k p :
-
Rate constant of the peeling reaction
- k s :
-
Rate constant of the stopping reaction
- MALLS:
-
Multi-angle laser light scattering
- MSA:
-
Metasaccharinic acid
- MB:
-
Methylene blue
- MMD:
-
Molar mass distribution
- Mn:
-
Number-average molecular weight
- Mw:
-
Weight-average molecular weight
- Odp:
-
Oven dried pulp
- P :
-
Mole fraction of peeled-off material
- PAD:
-
Pulsed amperometric detection
- PDI:
-
Polydispersity index
- R :
-
Amount of reducing end groups
- R 0 :
-
Initial reducing end group mole fraction
- REG:
-
Reducing end group
- SEC:
-
Size exclusion chromatography
- TCF:
-
Total chlorine free
- WRV:
-
Water retention value
- Γ0 :
-
Initial amount of the carbohydrates
- η:
-
Intrinsic viscosity
References
Barthel P, Philipp B (1967) Untersuchungen zum Abbauverlauf bei der Alkalicellulose-Vorreife verschiedenartiger Zellstoffe. Faserforschung und Textiltechnik 18(6):266–273 (537–538)
Bohrn R, Potthast A, Schiehser S, Rosenau T, Sixta H, Kosma P (2006) The FDAM method: determination of carboxyl profiles in dellulosic materials by combining group-selective fluorescence labeling with GPC. Biomacromolecules 7(6):1743–1750
Bywater N (2011) The global viscose fibre industry in the 21st century—the first 10 years. Lenzinger Berichte 89:22–29
Colbran RL, Davidson GF (1961) The degradative action of hot dilute alkalis on hydrocellulose. J Text Inst 52:T73–T87
Davidson GF (1934a) The dissolution of chemically modified cotton cellulose in alkaline solutions. Part I—in solutions of sodium hydroxide particularly at temperatures below the mormal. J Text Inst 25:T174–T196
Davidson GF (1934b) The dissolution of chemically modified cotton cellulose in alkaline solutions. Part I—in solutions of sodium hydroxide particularly at temperatures below the mormal. J Text Inst 25:T174–T196
Eichinger D (2012) A vision of the world of cellulosic fibers in 2020. Lenzinger Berichte 90:1–7
Entwistle D, Cole EH, Wooding NS (1949) The autoxidation of alkali cellulose. Part I: an experimental study of the kinetics of the reaction. Text Res J 19(9):527–546
Franzon O, Samuelson O (1957) Degradation of cellulose by alkali cooking. Sven Papperstidn 60(23):872–877
Glaus MA, Van Loon LR (2004) Technical report 03–08: cellulose degradation at alkaline conditions: long-term experiments at elevated temperatures, Hannover
Glaus MA, Van Loon LR (2008) Degradation of cellulose under alkaline conditions: new insights from a 12 years degradation study. Environ Sci Technol 42:2906–2911
Glaus MA, Van Loon LR, Achatz S, Chodura A, Fischer K (1999) Degradation of cellulosic materials under the alkaline conditions of a cementitious repository for low and intermediate level radioactive waste Part I: identification of degradation products. Anal Chim Acta 398:111–122
Götze K (1967) Chemiefasern nach dem Viskoseverfahren. Springer, Berlin
Haas DW, Hrutfiord BF, Sarkanen KV (1967) Kinetic study on the alkaline degradation of cotton hydrocellulose. J Appl Polym Sci 11:587
Hämmerle FM (2011) The cellulose gap (the future of cellulose fibres). Lenzinger Berichte 89:12–21
Johansson MH, Samuelson O (1975) End-wise degradation of hydrocellulose during hot alkali treatment. J Appl Polym Sci 19:3007–3013
Kolpak FJ, Weih M, Blackwell J (1978) Mercerization of cellulose: 1. Determination of the structure of mercerized cotton. Polymer 19(2):123–131
Krässig HA (1993) Cellulose: structure, accessibility and reactivity; methods of activation. Polymer monographs, vol 11. Gordon and Breach Science Publishers, Yverdon, Switzerland
Lai YZ (1981) In: International symposium on wood and pulping chemistry, Stockholm, The Ekman Days, vol 2. pp 26–33
Lai YZ (1996) Reactivity and accessibility of cellulose, hemicelluloses, and lignins. In: Hon DN-S (ed) Chemical modification of lignocellulosic materials. Marcel Dekker, New York, pp 35–95
Lai YZ, Ontto DE (1979) Effects of alkalinity on endwise depolymerisation of hydrocellulose. J Appl Polym Sci 23:3219–3225
Lai Y-Z, Sarkanen KV (1967) Kinetics of alkaline hydrolysis of glycosidic bonds in cotton cellulose. Cellul Chem Technol 1:517–527
Lai Y-Z, Sarkanen KV (1969) Kinetic study on the alkaline degradation of amylose. J Polym Sci C 28:15–26
Machell G, Richards GN (1957) The alkaline degradation of polysaccharides. Part II. The alkali-stable residue from the action of sodium hydroxide on cellulose. J Chem Soc 4500–4506
Mais U, Sixta H (2004) Characterization of alkali-soluble hemicelluloses of hardwood dissolving pulps. In: ACS Symposium Series, vol 864 (Hemicelluloses), pp 94–107
Nevell TP, Zeronian SH (eds) (1985) Cellulose chemistry and its applications. Ellis Horwood, Chichester
Niemelä K, Sjöström E (1986) The conversion of cellulose into carboxylic acids by a drastic alkali treatment. Biomass 11:215–221
Okamoto T, Meshitsuka G (2010) The nanostructure of kraft pulp 1: evaluation of various mild drying methods using field emission scanning electron microscopy. Cellulose 17:1171–1182
Paananen M, Tamminen T, Nieminen K, Sixta H (2010) Galactoglucomannan stabilization during the initial kraft cooking of scots pine. Holzforschung 64:683–692
Pavasars I, Hagberg J, Borén H, Allard B (2003) Alkaline degradation of cellulose: mechanisms and kinetics. J Polym Environ 11(2):1015–2636
Philipp B, Rehder W, Lang H (1965) Zur Carboxylbestimmung in Chemiezellstoffen. Das Papier 19(1):1–9
Potthast A, Rosenau T, Kosma P (2006) Analysis of oxidized functionalities in cellulose. Adv Polym Sci 205:1–48
Richards GN, Sephton HH (1957) The alkaline degradation of polysaccharides. Part I. Soluble products of the action of sodium hydroxide on cellulose. J Chem Soc 4492–4499
Richtzenhain H, Lindgren BO, Abrahamsson B, Holmberg K (1954) Über den alkalischen Abbau von Polysacchariden. I. Mitteil. Abbau von Baumwollhydrocellulose. Svensk Papperstidning 57(10):363–366
Röhrling J, Potthast A, Rosenau T, Sixta H, Kosma P (2002) Determination of carbonyl functions in cellulosic substrates. Lenzinger Berichte 81:89–97
Schelosky N, Röder T, Baldinger T, Milacher W, Morgenstern B, Sixta H (1999) Molecular mass distribution of cellulosic products by size exclusion chromatography in DMAc/LiCl. Das Papier 12(12):728–738
Schild G, Sixta H (2011) Sulfur-free dissolving pulps and their application for viscose and lyocell. Cellulose 18:1113–1128
Shen L, Patel MK (2010) Life cycle assessment of man-made cellulose fibres. Lenzinger Berichte 88:1–59
Sixta H, Schelosky N, Milacher W, Baldinger T, Röder T (2001) Characterization of alkali-soluble pulp fractions by chromatography. 11th ISWPC, vol 3. Nice, France
Sjöström E (1977) The behavior of wood polysaccharides during alkaline pulping processes. Tappi 60(9):151–157
Sjöström E (1991) Carbohydrate degradation products from alkaline treatment of biomass. Biomass Bioenergy 1(1):61–64
Tatevosyan EL, Makarova TP, Meos AI (1965) Characteristics of alkali cellulose obtained by a continuous process. Khim Volokna 4:26–29
Testova L, Nieminen K, Penttilä PA, Serimaa R, Potthast A, Sixta H (2013) Cellulose degradation in alkaline media upon acidic pretreatment and stabilisation. Carbohydr Polym. doi:10.1016/j.carbpol.2013.01.093
Van Loon LR, Glaus MA (1997) Review of the kinetics of alkaline degradation of cellulose in view of its relevance for safety assessment of radioactive waste repositories. J Environ Polym Degrad 5(2):97–109
Van Loon LR, Glaus MA, Laube A, Stallone S (1999) Degradation of cellulosic materials under the alkaline conditions of a cementitious repository for low- and intermediate-level radioactive waste. II. Degradation kinetics. J Environ Polym Degrad 7(1):41–51
Whistler RL (1963) Methods in carbohydrate chemistry, vol 3. Academic Press, New York
Wollboldt RP, Zuckerstätter G, Weber HK, Larsson PT, Sixta H (2010) Accessibility, reactivity and supramolecular structure of E. globulus pulps with reduced xylan content. Wood Sci Technol 44:533–546
Young RA, Sarkanen KV, Johnson G (1972) Marine plant polymers Part III. A kinetic analysis of the alkaline degradation of the polysaccharides with specific reference to (1→3)-beta-D-glucans. Carbohyd Res 21:111–122
Acknowledgments
Financial support was provided by the Austrian government, the provinces of lower Austria, upper Austria, and Carinthia as well as by Lenzing AG. We also express our gratitude to the Johannes Kepler University, Linz, the University of Natural Resources and Applied Life Sciences, Vienna, and Lenzing AG for their in-kind contributions
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mozdyniewicz, D.J., Nieminen, K. & Sixta, H. Alkaline steeping of dissolving pulp. Part I: cellulose degradation kinetics. Cellulose 20, 1437–1451 (2013). https://doi.org/10.1007/s10570-013-9926-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9926-2