Abstract
Chronic inflammation (CI) is a primary contributing factor involved in multiple diseases like cancer, stroke, diabetes, Alzheimer’s disease, allergy, asthma, autoimmune diseases, coeliac disease, glomerulonephritis, sepsis, hepatitis, inflammatory bowel disease, reperfusion injury, and transplant rejections. Despite several expansions in our understanding of inflammatory disorders and their mediators, it seems clear that numerous proteins participate in the onset of CI. One crucial protein pyruvate kinase M2 (PKM2) much studied in cancer is also found to be inextricably woven in the onset of several CI’s. It has been found that PKM2 plays a significant role in several disorders using a network of proteins that interact in multiple ways. For instance, PKM2 forms a close association with epidermal growth factor receptors (EGFRs) for uncontrolled growth and proliferation of tumor cells. In neurodegeneration, PKM2 interacts with apurinic/apyrimidinic endodeoxyribonuclease 1 (APE1) to onset Alzheimer’s disease pathogenesis. The cross-talk of protein tyrosine phosphatase 1B (PTP1B) and PKM2 acts as stepping stones for the commencement of diabetes. Perhaps PKM2 stores the potential to unlock the pathophysiology of several diseases. Here we provide an overview of the notoriously convoluted biology of CI’s and PKM2. The cross-talk of PKM2 with several proteins involved in stroke, Alzheimer’s, cancer, and other diseases has also been discussed. We believe that considering the importance of PKM2 in inflammation-related diseases, new options for treating various disorders with the development of more selective agents targeting PKM2 may appear.
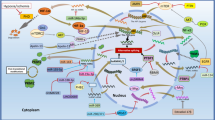
Graphical abstract








Similar content being viewed by others
Abbreviations
- 3PO:
-
3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one
- AD:
-
Alzheimer’s disease
- ADP:
-
adenosine diphosphate
- Akt:
-
protein kinase B or PKB
- AMPK:
-
adenosine monophosphate-activated protein kinase
- Ang-1:
-
angiopoietin-1
- APE1:
-
apurinic/apyrimidinic endodeoxyribonuclease 1
- ApoE:
-
apolipoprotein E
- ATP:
-
adenosine triphosphate
- Bcl-2:
-
B cell lymphoma 2
- Bcl-xl:
-
B cell lymphoma-extra large
- Bcr-Abl:
-
breakpoint cluster region - Abelson gene (fusion of gene) sequence
- Bmal:
-
brain and muscle ARNT-like protein
- BRAF:
-
proto-oncogene B-Raf & v-Raf murine sarcoma viral oncogene homolog B, serine/threonine-protein kinase B-Raf
- CAD:
-
coronary artery disease
- CCI:
-
chronic constriction injury
- CCL:
-
chemokine (c-c motif) ligand
- CD:
-
Crohn’s disease
- CDK4:
-
cyclin-dependant kinase 4
- CI:
-
chronic inflammation
- CNS:
-
central nervous system
- COL2A1:
-
collagen type 2 alpha 1 chain
- CRAF:
-
RAF proto-oncogene serine/threonine-protein kinase
- CSC:
-
cancer stem cell
- CUL3:
-
Cullin-3
- CXCL:
-
chemokine (C-X-C motif) ligand
- CXCR4:
-
C-X-C chemokine receptor type 4
- Cys:
-
3-letter code for amino acid cysteine
- DAPK:
-
death-associated protein kinase
- DBD:
-
DNA-binding domain
- DNA:
-
deoxyribonucleic acid
- DRAK:
-
death-associated protein kinase related
- DU145:
-
a human prostate cancer cell line
- EGF:
-
epidermal growth factor
- EGFR:
-
epidermal growth factor receptor
- EIF2AK2:
-
eukaryotic translation initiation factor 2-alpha kinase 2
- EMT:
-
epithelial-mesenchymal transitions
- e-NOS:
-
endothelial Nitric oxide synthase
- EPO:
-
erythropoietin
- ERK:
-
extracellular signal-regulated kinases
- EVs:
-
extracellular vesicles
- FAK:
-
focal adhesion kinase
- FDA or USFDA:
-
United States Food & Drug Administration
- FH:
-
fumarate dehydrogenase
- G12V:
-
glycine is mutated by Valine at the residue no. 12
- GLUT:
-
glucose transporter
- GM-CSF:
-
granulocyte-macrophage colony-stimulating factor
- GSK:
-
glycogen synthase kinase
- GTPase:
-
guanosine triphosphatase
- HER2:
-
human epidermal growth factor receptor 2
- HHcy:
-
hyperhomocysteinemia
- HI:
-
hypoxia-Ischemia
- HIF:
-
hypoxia including factor
- HMGB1:
-
high mobility group box 1 protein
- HRE:
-
hypoxia-responsive element
- HSP:
-
heat shock proteins
- I2H:
-
in silico 2-hybrid
- IBD:
-
inflammatory bowel disease
- ICAM-1:
-
intercellular adhesion molecule 1
- IFN:
-
interferon
- IGF:
-
insulin-like growth factor
- IgG:
-
immunoglobulin G
- IKKβ:
-
inhibitor of nuclear factor kappa-B kinase subunit beta
- IL:
-
interleukin
- iNOS:
-
inducible nitric oxide synthase
- JMJD5:
-
jumonji C domain-containing deoxygenase 5 protein
- JNK:
-
c-Jun N-terminal kinase
- K433:
-
1-letter code for amino acid lysine including its residue no. 433
- LDHA, LDA-A:
-
lactate dehydrogenase A
- LKB1:
-
liver kinase B1
- LPS:
-
lipopolysaccharide
- MAK:
-
mitogen-activated protein kinases (MAP kinase/MAPK)
- MCP:
-
monocyte chemoattractant protein
- MCT:
-
monocarboxylate transporter
- MDA-MB-231:
-
an epithelial human breast cancer cell line
- MDSC:
-
myeloid-derived suppressor cells
- MEK:
-
a type of serine/tyrosine/threonine kinase & one of the mitogen-activated kinases
- MET:
-
tyrosine-protein kinase Met or hepatocyte growth factor receptor (HGFR)
- MLC:
-
myosin light chain
- mRNA:
-
messenger RNA
- mtDNA:
-
mitochondrial DNA
- mTOR:
-
mammalian target for Rapamycin or mechanistic target for Rapamycin
- mTORC1:
-
mammalian target for Rapamycin complex 1 or mechanistic target for Rapamycin complex 1
- nDNA:
-
nuclear DNA
- NFκB:
-
nuclear factor kappa B
- NOX4:
-
NADPH oxidase 4
- OA:
-
osteoarthritis
- Oct:
-
octamer-binding transcription factor
- OSM:
-
oncostatin M
- OXPHOS:
-
oxidative phosphorylation
- P2X receptors:
-
purinergic receptors X
- P2Y receptors:
-
purinergic receptors Y
- p300:
-
epigenetic cofactor
- P-38:
-
a class of mitogen-activated protein kinases
- PAMP:
-
pathogen-associated molecular pattern
- PDAC:
-
pancreatic ductal adenocarcinoma
- PDK:
-
pyruvate dehydrogenase kinase
- PEP:
-
phosphoenolpyruvate
- PFKFB3:
-
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- PGHS:
-
prostaglandin endoperoxide H synthase
- PHD:
-
prolyl hydroxylase
- PI3K:
-
phosphoinositide 3-kinase or phosphatidylinositol 3-kinase
- PK:
-
pyruvate kinase
- PKC:
-
protein kinase C
- PKL:
-
pyruvate kinase L isoform
- PKLR:
-
pyruvate kinase LR gene
- PKM:
-
pyruvate kinase M gene
- PKM1:
-
pyruvate kinase M1 isoform
- PKM2:
-
pyruvate kinase M2 isoform
- PKR:
-
pyruvate kinase R isoform
- PPI:
-
protein–protein interaction
- PPP:
-
pentose phosphate pathway
- PROTAC:
-
proteolysis targeting chimera
- PRR:
-
pattern recognition receptors
- PsA:
-
psoriatic arthritis
- PTB:
-
polypyrimidine tract-binding protein
- PTP1B:
-
protein–tyrosine phosphatase 1B
- RA:
-
rheumatoid arthritis
- Ral (RalA and RalB):
-
Ras-related protein RalA and RalB
- RASFC:
-
rheumatoid arthritis-synovial fibroblast cells
- Ras-Raf-MEK-ERK pathway:
-
also known as MAPK/ERK pathway
- RET/PTC1:
-
rearranged during transfection/papillary thyroid carcinoma 1
- RNA:
-
ribonucleic acid
- ROCK2:
-
Rho-associated protein kinase
- ROS:
-
reactive oxygen species
- S15:
-
1-letter code for amino acid Serine including its residue no. 15
- SDF:
-
stromal cell–derived factor
- SDH:
-
succinate dehydrogenase
- Ser:
-
3-letter code for amino acid Serine
- SOX-9:
-
a transcription factor
- STAT:
-
signal transducer and activator of transcription proteins
- SUMOlyation:
-
small ubiquitin-like modification
- T45:
-
1-letter code for amino acid Threonine including its residue no. 45
- TAD:
-
transcriptional activation domain
- TAP-MS:
-
tandem affinity purification-mass spectroscopy
- TBK1:
-
TANK-binding kinase 1
- TCA:
-
tricarboxylic acid
- TGFIF2:
-
TGF-β induced factor homeobox-2
- TGF-β :
-
transforming growth factor β
- Tie-2:
-
angiopoietin-1 receptor
- TLR:
-
Toll-like receptors
- TNF:
-
tumour necrosis factor
- Tyr:
-
3-letter code of amino acid tyrosine
- U251:
-
a human brain cell line
- UC:
-
ulcerative colitis
- VCAM-1:
-
vascular cell adhesion protein 1
- VEGF:
-
vascular endothelial growth factor
- Y105F:
-
tyrosine is mutated with phenylalanine at the residue no. 105
- Y118:
-
1-letter code for amino acid tyrosine including its residue no. 118
- Y2H:
-
yeast 2-hybrid
- Y333:
-
1-letter code of amino acid tyrosine including its residue no. 333
- αSyn:
-
α-synuclein
References
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. Elsevier. 2000;21(3):383–421.
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell 4th ed. New York: Garland Science; 2002.
Almousa AA, Morris M, Fowler S, Jones J, Alcorn J. Elevation of serum pyruvate kinase M2 (PKM2) in IBD and its relationship to IBD indices. Clin Biochem. Elsevier. 2018;53:19–24.
Alquraishi M, Puckett DL, Alani DS, Humidat AS, Frankel VD, Donohoe DR, et al. Pyruvate kinase M2: a simple molecule with complex functions. Free Radic Biol Med. Elsevier. 2019;143:176–92.
Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics Elsevier. 2004;84(6):1014–20.
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang J, Shen M, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science (80-. ). [Internet]. American Association for the Advancement of Science. 2011;334(6060):1278–83 Available from: https://www.ncbi.nlm.nih.gov/books/NBK153222/.
Ando M, Uehara I, Kogure K, Asano Y, Nakajima W, Abe Y, et al. Interleukin 6 enhances glycolysis through expression of the glycolytic enzymes hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3. J. Nippon Med. Sch. The Medical Association of Nippon Medical School. 2010;77(2):97–105.
Angiari S, Runtsch MC, Sutton CE, Palsson-McDermott EM, Kelly B, Rana N, et al. Pharmacological activation of pyruvate kinase M2 inhibits CD4+ T cell pathogenicity and suppresses autoimmunity. Cell Metab. Elsevier. 2020;31(2):391–405.
Ashok BS, Ajith TA, Sivanesan S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. Wiley Online Library. 2017;44(3):327–34.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. Elsevier. 2001;357(9255):539–45.
Barnett JA. A history of research on yeasts 5: the fermentation pathway. Yeast. John Wiley & Sons, Ltd. Chichester, UK. 2003;20(6):509–43.
Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med J Saudi Medical Journal. 2015;36(3):273–9.
Baselga J, Albanell J. Epithelial growth factor receptor interacting agents. Hematol Oncol Clin North Am. 2002;16(5):1041–63.
Bettaieb A, Bakke J, Nagata N, Matsuo K, Xi Y, Liu S, et al. Protein tyrosine phosphatase 1B regulates pyruvate kinase M2 tyrosine phosphorylation. J Biol Chem. ASBMB. 2013;288(24):17360–71.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. Elsevier. 2015;162(3):540–51.
Blaikie L, Kay G, Lin PKT. Current and emerging therapeutic targets of Alzheimer’s disease for the design of multi-target directed ligands. Medchemcomm Royal Society of Chemistry. 2019;10(12):2052–72.
Borst K, Schwabenland M, Prinz M. Microglia metabolism in health and disease. Neurochem Int. Elsevier. 2019;130:104331.
Boudewijn MT, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature Nature Publishing Group. 1995;376(6541):599–602.
Brown JB, Cheresh P, Goretsky T, Managlia E, Grimm GR, Ryu H, et al. Epithelial PI3K signaling is required for β-catenin activation and host defense against Citrobacter rodentium infection. Infect Immun Am Soc Microbiol. 2011.
Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. IOS Press. 2010;26(1):5–13.
Butterfield DA, Abdul HM, Opii W, Newman SF, Joshi G, Ansari MA, et al. Pin1 in Alzheimer’s disease. J Neurochem. Wiley Online Library. 2006;98(6):1697–706.
Canal F, Perret C. PKM2: a new player in the β-catenin game. Futur Oncol Future Medicine. 2012;8(4):395–8.
Cartier N, Lewis C-A, Zhang R, Rossi FMV. The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol Springer. 2014;128(3):363–80.
Chakrabarti R. Transcriptional regulation of the rat glutamine synthetase gene by tumor necrosis factor-α. Eur J Biochem Wiley Online Library. 1998;254(1):70–4.
Chatterjee S, Burns TF. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int. J. Mol. Sci. Multidisciplinary Digital Publishing Institute. 2017;18(9):1978.
Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. Elsevier. 2013;108:21–43.
Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr Springer. 2007;39(3):267–74.
Chen L, Tang Z, Wang X, Ma H, Shan D, Cui S. PKM2 aggravates palmitate-induced insulin resistance in HepG2 cells via STAT3 pathway. Biochem Biophys Res Commun. Elsevier. 2017;492(1):109–15.
Chen D, Wei L, Liu Z-R, Yang JJ, Gu X, Wei ZZ, et al. Pyruvate kinase M2 increases angiogenesis, neurogenesis, and functional recovery mediated by upregulation of STAT3 and focal adhesion kinase activities after ischemic stroke in adult mice. Neurotherapeutics. Springer. 2018a;15(3):770–84.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. Impact Journals, LLC. 2018b;9(6):7204.
Cortés-Cros M, Hemmerlin C, Ferretti S, Zhang J, Gounarides JS, Yin H, et al. M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth. Proc. Natl. Acad. Sci. National Acad Sciences. 2013;110(2):489–94.
Costantini LC, Barr LJ, Vogel JL, Henderson ST. Hypometabolism as a therapeutic target in Alzheimer’s disease. BMC Neurosci. Springer. 2008;9(S2):S16.
Dang CV. PKM2 tyrosine phosphorylation and glutamine metabolism signal a different view of the Warburg effect. Sci. Signal. American Association for the Advancement of Science. 2009;2(97):pe75–pe75.
Dayton TL, Gocheva V, Miller KM, Israelsen WJ, Bhutkar A, Clish CB, et al. Germline loss of PKM2 promotes metabolic distress and hepatocellular carcinoma. Genes Dev Cold Spring Harbor Lab. 2016;30(9):1020–33.
De Las Rivas J, Fontanillo C. Protein–protein interactions essentials: key concepts to building and analyzing interactome networks. PLoS Comput Biol. Public Library of Science. 2010;6(6):e1000807.
Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. Elsevier. 2010;17(3):197–218.
Demetrius LA, Magistretti PJ, Pellerin L. Alzheimer’s disease: the amyloid hypothesis and the Inverse Warburg effect. Front. Physiol. Frontiers. 2015;5:522.
Deng J, Lü S, Liu H, Liu B, Jiang C, Xu Q, et al. Homocysteine activates B cells via regulating PKM2-dependent metabolic reprogramming. J Immunol Am Assoc Immnol. 2017;198(1):170–83.
Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang M, et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. Elsevier. 2018;24(2):366–78.
Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. ACS Publications. 2005;44(27):9417–29.
Dorababu A. Critical evaluation of current Alzheimer’s drug discovery (2018–19) & futuristic Alzheimer drug model approach. Bioorg Chem. Elsevier. 2019;93:103299.
Erol A. Death-associated proliferation kinetic in normal and transformed cells. Cell cycle Taylor & Francis. 2012;11(8):1512–6.
Everts B, Amiel E, Huang SC-C, Smith AM, Chang C-H, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol. Nature Publishing Group. 2014;15(4):323–32.
Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. Dove Press. 2014;7:113.
Fan X, Pei S, Zhou D, Zhou P, Huang Y, Hu X, et al. Melittin ameliorates inflammation in mouse acute liver failure via inhibition of PKM2-mediated Warburg effect. Acta Pharmacol Sin. Nature Publishing Group. 2020:1–11.
Fantin VR, Berardi MJ, Scorrano L, Korsmeyer SJ, Leder P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell. Elsevier. 2002;2(1):29–42.
Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. Elsevier. 2006;9(6):425–34.
Farag AK, Roh EJ. Death-associated protein kinase (DAPK) family modulators: current and future therapeutic outcomes. Med Res Rev. Wiley Online Library. 2019;39(1):349–85.
Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. Nature Publishing Group. 2009;6(5):297–305.
Feng J, Wu L, Ji J, Chen K, Yu Q, Zhang J, et al. PKM2 is the target of proanthocyanidin B2 during the inhibition of hepatocellular carcinoma. J Exp Clin Cancer Res BioMed Central. 2019;38(1):1–15.
Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science (80-. ). American Association for the Advancement of Science. 1987;235(4795):1492–5.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. Nature Publishing Group. 2004;4(11):891–9.
Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin. Sci. Portland Press Ltd. 2018;132(12):1243–52.
Gomes MA, Priolli DG, Tralhao JG, Botelho MF. Hepatocellular carcinoma: epidemiology, biology, diagnosis, and therapies. Rev. da Assoc. Médica Bras. (English Ed. Elsevier). 2013;59(5):514–24.
Gui DY, Lewis CA, Vander Heiden MG. Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci. Signal. American Association for the Advancement of Science. 2013;6(263):pe7–pe7.
Hall CN, Klein-Flügge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci Soc Neuroscience. 2012;32(26):8940–51.
Hamabe A, Konno M, Tanuma N, Shima H, Tsunekuni K, Kawamoto K, et al. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial–mesenchymal transition. Proc. Natl. Acad. Sci. National Acad Sciences. 2014;111(43):15526–31.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. Elsevier. 2000;100(1):57–70.
Hanlon MM, Rakovich T, Cunningham CC, Ansboro S, Veale DJ, Fearon U, et al. STAT3 Mediates the differential effects of oncostatin M and TNFα on RA synovial fibroblast and endothelial cell function. Front Immunol. Frontiers. 2019;10:2056.
Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. The Rockefeller University Press. 2018;217(2):459–72.
Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer Nature Publishing Group. 2002;2(1):38–47.
Hartmann S, Ridley AJ, Lutz S. The function of Rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol. Frontiers. 2015;6:276.
Hewick RM, Lu Z, Wang JH. Proteomics in drug discovery. Adv Protein Chem. Elsevier. 2003:309–42.
Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem Wiley Online Library. 2008;104(6):1433–9.
Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR. Vincent J-L. Sepsis and septic shock. Nat Rev Dis Prim. Nature Publishing Group. 2016;2(1):1–21.
Hou P, Luo L, Chen H, Chen Q, Bian X, Wu S, et al. Ectosomal PKM2 promotes HCC by inducing macrophage differentiation and remodeling the tumor microenvironment. Mol Cell. Elsevier. 2020;78(6):1192–206.
Huang L, Yu Z, Zhang T, Zhao X, Huang G. HSP40 interacts with pyruvate kinase M2 and regulates glycolysis and cell proliferation in tumor cells. PLoS One. Public Library of Science. 2014;9(3):e92949.
Huang J, Liu K, Zhu S, Xie M, Kang R, Cao L, et al. AMPK regulates immunometabolism in sepsis. Brain Behav Immun. Elsevier. 2018;72:89–100.
Iansante V, Choy PM, Fung SW, Liu Y, Chai J-G, Dyson J, et al. PARP14 promotes the Warburg effect in hepatocellular carcinoma by inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat Commun. Nature Publishing Group. 2015;6(1):1–15.
Inbal B, Shani G, Cohen O, Kissil JL, Kimchi A. Death-associated protein kinase-related protein 1, a novel serine/threonine kinase involved in apoptosis. Mol Cell Biol Am Soc Microbiol. 2000;20(3):1044–54.
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. Elsevier. 2013;155(2):397–409.
Jain T, Nikolopoulou EA, Xu Q, Qu A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol Ther. Elsevier. 2018;183:22–33.
Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. Elsevier. 2014a;53(1):75–87.
Jiang Y, Wang Y, Wang T, Hawke DH, Zheng Y, Li X, et al. PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat Commun. Nature Publishing Group. 2014b;5(1):1–14.
Jin K, Li T, Sánchez-Duffhues G, Zhou F, Zhang L. Involvement of inflammation and its related microRNAs in hepatocellular carcinoma. Oncotarget. Impact Journals, LLC. 2017;8(13):22145.
Jones S, Thornton JM. Principles of protein-protein interactions. Proc Natl Acad Sci. National Acad Sciences. 1996;93(1):13–20.
Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim. Biophys. Acta (BBA)-Bioenergetics. Elsevier. 2011;1807(6):552–61.
Katsumoto A, Takeuchi H, Takahashi K, Tanaka F. Microglia in Alzheimer’s disease: risk factors and inflammation. Front Neurol. Frontiers. 2018;9:978.
Kawai T, Nomura F, Hoshino K, Copeland NG, Gilbert DJ, Jenkins NA, et al. Death-associated protein kinase 2 is a new calcium/calmodulin-dependent protein kinase that signals apoptosis through its catalytic activity. Oncogene. Nature Publishing Group. 1999;18(23):3471–80.
Keller KE, Tan IS, Lee Y-S. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science (80-. ). American Association for the Advancement of Science. 2012;338(6110):1069–72.
Keller KE, Doctor ZM, Dwyer ZW, Lee Y-S. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell. Elsevier. 2014;53(5):700–9.
Kim AS, Johnston SC. Temporal and geographic trends in the global stroke epidemic. Stroke Am Heart Assoc. 2013;44(6_suppl_1):S123–S5.
Knowles JR. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. Annual Reviews 4139 El Camino Way, PO Box 10139, Palo Alto, CA 94303-0139, USA. 1980;49(1):877–919.
KoÈgel D, PloÈttner O, Landsberg G, Christian S, Scheidtmann KH. Cloning and characterization of Dlk, a novel serine/threonine kinase that is tightly associated with chromatin and phosphorylates core histones. Oncogene. 1998;17(20).
Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. Taylor & Francis. 2006;38(3):200–11.
Kong Q, Li N, Cheng H, Zhang X, Cao X, Qi T, et al. HSPA12A is a novel player in nonalcoholic steatohepatitis via promoting nuclear PKM2-mediated M1 macrophage polarization. Diabetes Am Diabetes Assoc. 2019;68(2):361–76.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. Nature Publishing Group. 2011;11(5):325–37.
Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells, Mol Dis. Elsevier. 2004;32(1):52–7.
Kunze R, Zhou W, Veltkamp R, Wielockx B, Breier G, Marti HH. Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia. Stroke Am Heart Assoc. 2012;43(10):2748–56.
Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell Elsevier. 2007;130(4):691–703.
Lee J, Kim HK, Han Y-M, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol Elsevier. 2008;40(5):1043–54.
Lee H, Jeong AJ, Ye S-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. Korean Society for Biochemistry and Molecular Biology. 2019;52(7):415.
Lesley J, Hyman R, English N, Catterall JB, Turner GA. CD44 in inflammation and metastasis. Glycoconj J Springer. 1997;14(5):611–22.
Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. Elsevier. 1998:49–139.
Li X-J, Xu M, Zhao X-Q, Zhao J-N, Chen F-F, Yu W, et al. Proteomic analysis of synovial fibroblast-like synoviocytes from rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(4):552–8.
Li L, Zhu L, Hao B, Gao W, Wang Q, Li K, et al. iNOS-derived nitric oxide promotes glycolysis by inducing pyruvate kinase M2 nuclear translocation in ovarian cancer. Oncotarget. Impact Journals, LLC. 2017;8(20):33047.
Li L, Tang L, Yang X, Chen R, Zhang Z, Leng Y, et al. Gene regulatory effect of pyruvate kinase M2 is involved in renal inflammation in type 2 diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes. © Georg Thieme Verlag KG. 2020.
Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. Nature Publishing Group. 2017;27(3):329–51.
Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. Elsevier. 2016;41(3):211–8.
Liu W-R, Tian M-X, Yang L-X, Lin Y-L, Jin L, Ding Z-B, et al. PKM2 promotes metastasis by recruiting myeloid-derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget. Impact Journals, LLC. 2015;6(2):846.
Liu M, Wang Y, Ruan Y, Bai C, Qiu L, Cui Y, et al. PKM2 promotes reductive glutamine metabolism. Cancer Biol Med. Chinese Anti-Cancer Association. 2018;15(4):389.
Liu T, Li S, Wu L, Yu Q, Li J, Feng J, et al. Experimental study of hepatocellular carcinoma treatment by shikonin through regulating PKM2. J Hepatocell Carcinoma. Dove Press. 2020;7:19.
Lochmatter C, Fischer R, Charles PD, Yu Z, Powrie F, Kessler BM. Integrative phosphoproteomics links IL-23R signaling with metabolic adaptation in lymphocytes. Sci Rep. Nature Publishing Group. 2016;6(1):1–12.
Lü S, Deng J, Liu H, Liu B, Yang J, Miao Y, et al. PKM2-dependent metabolic reprogramming in CD4+ T cells is crucial for hyperhomocysteinemia-accelerated atherosclerosis. J Mol Med. Springer. 2018;96(6):585–600.
Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. Elsevier. 2015;57(1):95–107.
Luo W, Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget. Impact Journals, LLC. 2011;2(7):551.
Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. Elsevier. 2011;145(5):732–44.
Makinoshima H, Takita M, Saruwatari K, Umemura S, Obata Y, Ishii G, et al. Signaling through the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) axis is responsible for aerobic glycolysis mediated by glucose transporter in epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma. J Biol Chem. ASBMB. 2015;290(28):17495–504.
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. American Society of Hematology. 2005;105(2):659–69.
Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. Taylor & Francis. 2012;10(6):701–6.
McGarry T, Biniecka M, Gao W, Cluxton D, Canavan M, Wade S, et al. Resolution of TLR2-induced inflammation through manipulation of metabolic pathways in rheumatoid arthritis. Sci Rep Nat. Publ Group. 2017;7:43165.
McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. Elsevier. 2011;15(2):223–43.
Meng X, Carlson NR, Dong J, Zhang Y. Oncogenic c-Myc-induced lymphomagenesis is inhibited non-redundantly by the p19Arf–Mdm2–p53 and RP–Mdm2–p53 pathways. Oncogene. Nature Publishing Group. 2015;34(46):5709–17.
Meng Y, Li H, Liu C, Zheng L, Shen B. Jumonji domain-containing protein family: the functions beyond lysine demethylation. J Mol Cell Biol. Oxford University Press. 2018;10(4):371–3.
Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. Elsevier. 2016;167(2):457–70.
Miranda-Gonçalves V, Reis RM, Baltazar F. Lactate transporters and pH regulation: potential therapeutic targets in glioblastomas. Curr. Cancer Drug Targets. Bentham Science Publishers. 2016;16(5):388–99.
Mishra A, Jaiswal A, Stahr N, Makhija S, Sandey M, Suryawanshi A. Pyruvate kinase M2 (PKM2)-mediated glycolytic upregulation in lung CD11c+ cells facilitates alternaria-induced acute airway inflammation. A31. ASTHMA Transl. Stud. American Thoracic Society. 2020:A1286–6.
Mittal S, El-Serag HB. Epidemiology of HCC: consider the population. J Clin Gastroenterol. NIH Public Access. 2013;47:S2.
Mor I, Carlessi R, Ast T, Feinstein E, Kimchi A. Death-associated protein kinase increases glycolytic rate through binding and activation of pyruvate kinase. Oncogene. Nature Publishing Group. 2012;31(6):683–93.
Morita M, Sato T, Nomura M, Sakamoto Y, Inoue Y, Tanaka R, et al. PKM1 confers metabolic advantages and promotes cell-autonomous tumor cell growth. Cancer Cell. Elsevier. 2018;33(3):355–67.
Nemoto S, Takeda K, Yu Z-X, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol Am Soc Microbiol. 2000;20(19):7311–8.
Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, et al. The Akt pathway in oncology therapy and beyond. Int J Oncol. Spandidos Publications. 2018;53(6):2319–31.
Noguchi T, Inoue H, Tanaka T. The M1-and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. ASBMB. 1986;261(29):13807–12.
Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. Springer. 2011;8(3):319–29.
Pages G, Lenormand P, L’Allemain G, Chambard J-C, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci. National Acad Sciences. 1993;90(18):8319–23.
Palsson-McDermott EM, O’neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. Wiley Online Library. 2013;35(11):965–73.
Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. Elsevier. 2015;21(1):65–80.
Papa S, Choy PM, Bubici C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene. Nature Publishing Group. 2019;38(13):2223–40.
Park YS, Kim DJ, Koo H, Jang SH, You Y-M, Cho JH, et al. AKT-induced PKM2 phosphorylation signals for IGF-1-stimulated cancer cell growth. Oncotarget. Impact Journals, LLC. 2016;7(30):48155.
Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Membr. anomalies tumor cells. Karger Publishers. 1978:190–274.
Peng Y, Xing S, Tang H, Wang C, Yi F, Liu G, et al. Influence of glucose transporter 1 activity inhibition on neuroblastoma in vitro. Gene. Elsevier. 2019;689:11–7.
Perwez Hussain S, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. Wiley Online Library. 2007;121(11):2373–80.
Pimenta RJ, Massabki PS. Hepatocellular carcinoma: a clinical outlook. Rev Bras Clin Med. 2010;8:59–67.
Qiao H, He X, Zhang Q, Yuan H, Wang D, Li L, et al. Alpha-synuclein induces microglial migration via PKM2-dependent glycolysis. Int J Biol Macromol. Elsevier. 2019;129:601–7.
Rahman MR, Islam T, Zaman T, Shahjaman M, Karim MR, Huq F, et al. Identification of molecular signatures and pathways to identify novel therapeutic targets in Alzheimer’s disease: insights from a systems biomedicine perspective. Genomics. Elsevier. 2020;112(2):1290–9.
Rao VS, Srinivas K, Sujini GN, Kumar GN. Protein-protein interaction detection: methods and analysis. Int J Proteomics. Hindawi. 2014;2014.
Ratter JM, Rooijackers HMM, Hooiveld GJ, Hijmans AGM, de Galan BE, Tack CJ, et al. In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front Immunol. Frontiers. 2018;9:2564.
Rezaei T, Amini M, Hashemi ZS, Mansoori B, Rezaei S, Karami H, et al. microRNA-181 serves as a dual-role regulator in the development of human cancers. Free Radic. Biol. Med. Elsevier. 2019.
Rihan M, Nalla LV, Dharavath A, Shard A, Kalia K, Khairnar A. Pyruvate kinase M2: a metabolic bug in re-wiring the tumor microenvironment. Cancer Microenviron. Springer. 2019:1–19.
Roiniotis J, Dinh H, Masendycz P, Turner A, Elsegood CL, Scholz GM, et al. Hypoxia prolongs monocyte/macrophage survival and enhanced glycolysis is associated with their maturation under aerobic conditions. J Immunol Am Assoc Immnol. 2009;182(12):7974–81.
Sahota P, Savitz SI. Investigational therapies for ischemic stroke: neuroprotection and neurorecovery. Neurotherapeutics. Springer. 2011;8(3):434–51.
Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. The Company of Biologists Ltd. 2010;123(7):1007–13.
Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. Wiley Online Library. 1995;9(9):726–35.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. Elsevier. 2005;7(1):77–85.
Selvi R. Role of SOX9 in the etiology of Pierre-Robin syndrome. Iran. J. Basic Med. Sci. Mashhad University of Medical Sciences. 2013;16(5):700.
Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. STKE. American Association for the Advancement of Science. 2007;2007(407):cm8–cm8.
Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell Elsevier. 2012;148(3):399–408.
Senthelal S, Thomas MA. Arthritis. StatPearls [Internet]: StatPearls Publishing; 2019.
Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets. Taylor & Francis. 2012;16(1):15–31.
Sevin M, Girodon F, Garrido C, De Thonel A. HSP90 and HSP70: implication in inflammation processes and therapeutic approaches for myeloproliferative neoplasms. Mediators Inflamm. Hindawi. 2015;2015.
Shang S, Hua F, Hu Z-W. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. Impact Journals, LLC. 2017;8(20):33972.
Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. The Rockefeller University Press. 2016;213(3):337–54.
Siragusa M, Thoele J, Bibli S, Luck B, Loot AE, de Silva K, et al. Nitric oxide maintains endothelial redox homeostasis through PKM 2 inhibition. EMBO J. 2019;38(17):e100938.
Skommer J, Wlodkowic D, Deptala A. Larger than life: mitochondria and the Bcl-2 family. Leuk Res. Elsevier. 2007;31(3):277–86.
Smolková K, Plecitá-Hlavatá L, Bellance N, Benard G, Rossignol R, Ježek P. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol. Elsevier. 2011;43(7):950–68.
Straub RH, Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. public Heal. Oxford University Press. 2016;2016(1):37–51.
Suganuma K, Miwa H, Imai N, Shikami M, Gotou M, Goto M, et al. Energy metabolism of leukemia cells: glycolysis versus oxidative phosphorylation. Leuk Lymphoma. Taylor & Francis. 2010;51(11):2112–9.
Takenaka M, Yamada K, Lu T, Kang R, Tanaka T, Noguchi T. Alternative splicing of the pyruvate kinase M gene in a minigene system. Eur J Biochem. Wiley Online Library. 1996;235(1-2):366–71.
Tang C-Y, Mauro C. Similarities in the metabolic reprogramming of immune system and endothelium. Front. Immunol. Frontiers. 2017;8:837.
Tang Q, Ji Q, Xia W, Li L, Bai J, Ni R, et al. Pyruvate kinase M2 regulates apoptosis of intestinal epithelial cells in Crohn’s disease. Dig Dis Sci. Springer. 2015;60(2):393–404.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. Nature Publishing Group. 2013;496(7444):238–42.
Tejera D, Heneka MT. Microglia in Alzheimer’s disease: the good, the bad and the ugly. Curr. Alzheimer Res. Bentham Science Publishers. 2016;13(4):370–80.
Temmerman K, Simon B, Wilmanns M. Structural and functional diversity in the activity and regulation of DAPK-related protein kinases. FEBS J. Wiley Online Library. 2013;280(21):5533–50.
Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes to cells. Wiley Online Library. 1998;3(11):697–707.
Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, Pavarino ÉC, da Silva RF. Hepatocellular carcinoma: a comprehensive review of biomarkers, clinical aspects, and therapy. Asian Pacific J. cancer Prev. APJCP. Shahid Beheshti University of Medical Sciences. 2017;18(4):863.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (80-. ). American Association for the Advancement of Science. 2009;324(5930):1029–33.
Wade SM, Ohnesorge N, McLoughlin H, Biniecka M, Carter SP, Trenkman M, et al. Dysregulated miR-125a promotes angiogenesis through enhanced glycolysis. EBioMedicine. Elsevier. 2019;47:402–13.
Walkowiak J, Banasiewicz T, Krokowicz P, Hansdorfer-Korzon R, Drews M, Herzig K-H. Fecal pyruvate kinase (M2-PK): a new predictor for inflammation and severity of pouchitis. Scand J Gastroenterol. Taylor & Francis. 2005;40(12):1493–4.
Wang H-J, Hsieh Y-J, Cheng W-C, Lin C-P, Lin Y, Yang S-F, et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α–mediated glucose metabolism. Proc Natl Acad Sci. National Acad Sciences. 2014;111(1):279–84.
Wang F, Wang K, Xu W, Zhao S, Ye D, Wang Y, et al. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Cell Rep. Elsevier. 2017;19(11):2331–44.
Wang B, Liu S, Fan B, Xu X, Chen Y, Lu R, et al. PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord. J Headache Pain BioMed Central. 2018;19(1):7.
Wang Q, Lu D, Fan L, Li Y, Liu Y, Yu H, et al. COX-2 induces apoptosis-resistance in hepatocellular carcinoma cells via the HIF-1α/PKM2 pathway. Int J Mol Med. Spandidos Publications. 2019;43(1):475–88.
Westermarck J, Ivaska J, Corthals GL. Identification of protein interactions involved in cellular signaling. Mol Cell Proteomics. ASBMB. 2013;12(7):1752–63.
Wiese EK, Hitosugi T. Tyrosine kinase signaling in cancer metabolism: PKM2 paradox in the warburg effect. Front. Cell Dev. Biol. Frontiers. 2018;6:79.
Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. Elsevier. 2010;35(3):161–8.
Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. Nature Publishing Group. 2016;7(1):1–13.
Xu R, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. AACR. 2005;65(2):613–21.
Yang W, Lu Z. Nuclear PKM2 regulates the Warburg effect. Cell cycle. Taylor & Francis. 2013;12(19):3343–7.
Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. Nature Publishing Group. 2011;480(7375):118–22.
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L, et al. EGFR-induced and PKCε monoubiquitylation-dependent NF-κB activation upregulates PKM2 expression and promotes tumorigenesis. Mol Cell. Elsevier. 2012a;48(5):771–84.
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. Nature Publishing Group. 2012b;14(12):1295–304.
Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. Nature Publishing Group. 2014;5(1):1–9.
Yang P, Li Z, Li H, Lu Y, Wu H, Li Z. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell Signal. Elsevier. 2015;27(7):1525–32.
Yang L, Wang H, Liu L, Xie A. The role of insulin/IGF-1/PI3K/Akt/GSK3β signaling in parkinson’s disease dementia. Front. Neurosci. Frontiers. 2018a;12:73.
Yang X, Chen W, Zhao X, Chen L, Li W, Ran J, et al. Pyruvate kinase M2 modulates the glycolysis of chondrocyte and extracellular matrix in osteoarthritis. DNA Cell Biol. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA. 2018b;37(3):271–7.
Yang J, Dang G, Lü S, Liu H, Ma X, Han L, et al. T-cell–derived extracellular vesicles regulate B-cell IgG production via pyruvate kinase muscle isozyme 2. FASEB J. Wiley Online Library. 2019;33(11):12780–99.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. cell Biol. Nature Publishing Group. 2001;2(2):127–37.
Ye J, Mancuso A, Tong X, Ward PS, Fan J, Rabinowitz JD, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. National Acad Sciences. 2012;109(18):6904–9.
Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. Nature Publishing Group. 2016;35(15):1926–42.
Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia. Mol Med Rep. Spandidos Publications. 2018;18(4):3547–54.
Zhao X, Guan J-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev Elsevier. 2011;63(8):610–5.
Zhao X, Zhao L, Yang H, Li J, Min X, Yang F, et al. Pyruvate kinase M2 interacts with nuclear sterol regulatory element–binding protein 1a and thereby activates lipogenesis and cell proliferation in hepatocellular carcinoma. J Biol Chem Elsevier. 2018;293(17):6623–34.
Zhao P, Han S-N, Arumugam S, Yousaf MN, Qin Y, Jiang JX, et al. Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am. J. Physiol. Liver Physiol. American Physiological Society Bethesda, MD. 2019;317(4):G387–97.
Zheng JIE. Energy metabolism of cancer: glycolysis versus oxidative phosphorylation. Oncol Lett. Spandidos Publications. 2012;4(6):1151–7.
Zhou J, Yi Q, Tang L. The roles of nuclear focal adhesion kinase (FAK) on cancer: a focused review. J Exp Clin Cancer Res. Springer. 2019a;38(1):250.
Zhou Q, Xu J, Liu M, He L, Zhang K, Yang Y, et al. Warburg effect is involved in apelin-13-induced human aortic vascular smooth muscle cells proliferation. J Cell Physiol. Wiley Online Library. 2019b;234(9):14413–21.
Zhu R, Lei Y-Q, Zhao D-C. Overexpression of CXCL14 alleviates ventilator-induced lung injury through the downregulation of PKM2-mediated cytokine production. Mediators Inflamm. Hindawi. 2020;2020.
Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. Elsevier. 2004;313(3):459–65.
Acknowledgements
The authors gratefully acknowledge Director, NIPER-Ahmedabad for her support and encouragement. The communication number for publication is NIPER-A/438/5/2020. Authors SP, AD, PM, AS, AC, HJ, AD, DS, and LVN are thankful to NIPER-Ahmedabad, Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India for their fellowships.
Consent for publication
Not applicable
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
Dr. Amit Shard designed the concept of the protein–protein interaction and role in cancer. Sagarkumar Patel and Dr. Amit Shard wrote the role of PKM2 in chronic inflammation and cancer. Sagarkumar Patel, Anwesha Das, Payal Meshram, Ayushi Sharma, and Arnab Chowdhury helped in the literature search and data collection. Heena Jariyal and Dr. Akshay Srivastava wrote the role of glycolysis in cancer. Aishika Datta, Deepaneeta Sarmah, and Dr. Pallab Bhattacharya wrote the role of PKM2 in ischemic stroke. Lakshmi Vineela and Dr. Amit Khairnar wrote the role of the PKM2 in inflammatory diseases. Dr. Bichismita Sahu wrote the role of the PKM2 in Alzheimer’s disease. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, S., Das, A., Meshram, P. et al. Pyruvate kinase M2 in chronic inflammations: a potpourri of crucial protein–protein interactions. Cell Biol Toxicol 37, 653–678 (2021). https://doi.org/10.1007/s10565-021-09605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-021-09605-0