Abstract
During the process of catalytic conversion of CO2 to valuable chemical products, Pd used as catalysts or modifiers shows promising effect on CO2 hydrogenation. The mechanism of methanol synthesis from the hydrogenation of CO2 on the Pd(111) surface was studied using density functional theory calculations in present work. On the Pd(111) surface, CO2 firstly hydrogenates to HCOO or COOH, each of which then reacts with the surface H atom to form HCOOH. Next, HCOOH dissociates to OH and HCO that will be consecutively hydrogenated to H2CO, H3CO and H3COH. CO is the main side product of CO2 hydrogenation on the Pd(111) surface with an activation barrier of 1.09 eV. The hydrogenation of HCO species with the surface H atom to form H2CO plays as the rate determining step for CO2 hydrogenation to methanol with the barrier of 0.91 eV. Our calculated results are favorable for the understanding of the mechanism of CO2 conversion on not only Pd-based catalysts but also Pd modified catalysts.
Graphical Abstract
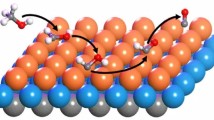
The mechanism of methanol synthesis from the hydrogenation of CO2 on the Pd(111) surface was studied using density functional theory calculations.









Similar content being viewed by others
References
Liu X-M, Lu GQ, Yan Z-F, Beltramini J (2003) Ind Eng Chem Res 42:6518
Palo DR, Dagle RA, Holladay JD (2007) Chem Rev 107:3992
Centi G, Perathoner S (2009) Catal Today 148:191
Masih D, Rohani S, Kondo JN, Tatsumi T (2017) Appl Catal B 217:247
Du X-L, Jiang Z, Su DS, Wang J-Q (2016) ChemSusChem 9:322
Liu L, Zhao C, Xu J, Li Y (2015) Appl Catal B 179:489
Jo D, Lim JB, Ryu T, Nam I-S, Camblor MA, Hong SB (2015) J Mater Chem 3:19322
Wang N, Shen K, Huang L, Yu X, Qian W, Chu W (2013) ACS Catal 3:1638
Ma J, Sun N, Zhang X, Zhao N, Xiao F, Wei W, Sun Y (2009) Catal Today 148:221
Delarmelina M, Marelli E, Carneiro JWdM, Nolan SP, Buhl M (2017) Chem Eur J 23:14954
Fan G, Zhao H, Duan Z, Fang T, Wan M, He L (2011) Catal Sci Technol 1:1138
Waugh KC (1992) Catal Today 15:51
Yang Y, Mims CA, Mei DH, Peden CHF, Campbell CT (2013) J Catal 298:10
Zhao Y-F, Yang Y, Mims C, Peden CHF, Li J, Mei D (2011) J Catal 281:199
Yang Y, Evans J, Rodriguez JA, White MG, Liu P (2010) PCCP 12:9909
Yang R, Zhang Y, Iwama Y, Tsubaki N (2005) Appl Catal A 288:126
Yang R, Yu X, Zhang Y, Li W, Tsubaki N (2008) Fuel 87:443
Yang R, Fu Y, Zhang Y, Tsubaki N (2004) J Catal 228:23
Toyir J, Piscina PR, Fierro JLG, Homs N (2001) Appl Catal B 29:207
Sloczynski J, Grabowski R, Olszewski P, Kozlowska A, Stoch J, Lachowska M, Skrzypek J (2006) Appl Catal A 310:127
Słoczyński J, Grabowski R, Kozłowska A, Olszewski P, Stoch J, Skrzypek J, Lachowska M (2004) Appl Catal A 278:11
Saito M, Fujitani T, Takeuchi M, Watanabe T (1996) Appl Catal A 138:311
Saito M, Fujitani T, Takahara I, Watanabe T, Takeuchi M, Kanai Y, Moriya K, Kakumoto T (1995) Energy Convers Manage 36:577
Ma Y, Sun Q, Wu D, Fan W-H, Zhang Y-L, Deng J-F (1998) Appl Catal A 171:45
Inui T, Hara H, Takeguchi T, Kim J-B (1997) Catal Today 36:25
Bonura G, Cordaro M, Cannilla C, Arena F, Frusteri F (2014) Appl Catal B 152–153:152
Arena F, Barbera K, Italiano G, Bonura G, Spadaro L, Frusteri F (2007) J Catal 249:185
Arakawa H, Dubois J-L, Sayama K (1992) Energy Convers Manage 33:521
Jiang X, Koizumi N, Guo X, Song C (2015) Appl Catal B 170–171:173
Choi EJ, Lee YH, Lee D-W, Moon D-J, Lee K-Y (2017) J Mol Catal 434:146
Melian-Cabrera I, Granados ML, Fierro JLG (2002) Catal Lett 79:165
Yang Y, White MG, Liu P (2011) J Phys Chem C 116:248
Ye J, Liu C-j, Mei D, Ge Q (2014) J Catal 317:44
Liang X-L, Dong X, Lin G-D, Zhang H-B (2009) Appl Catal B 88:315
Iwasa N, Suzuki H, Terashita M, Arai M, Takezawa N (2004) Catal Lett 96:75
Fan L, Fujimoto K (1995) Energy Convers Manage 36:633
Fan L, Fujimoto K (1994) J Catal 150:217
Collins SE, Chiavassa DL, Bonivardi AL, Baltanás MA (2005) Catal Lett 103:83
Rui N, Wang Z, Sun K, Ye J, Ge Q, Liu C-j (2017) Appl Catal B 218:488
Delley B (2000) J Chem Phys 113:7756
Delley B (1990) J Chem Phys 92:508
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Inada Y, Orita H (2008) J Comput Chem 29:225
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Harris IR, Norman M, Gardner WE (1972) J Alloys Compd 29:299
Halgren TA, Lipscomb WN (1977) Chem Phys Lett 49:225
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest about this article.
Rights and permissions
About this article
Cite this article
Zhang, M., Wu, Y., Dou, M. et al. A DFT Study of Methanol Synthesis from CO2 Hydrogenation on the Pd(111) Surface. Catal Lett 148, 2935–2944 (2018). https://doi.org/10.1007/s10562-018-2497-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2497-y