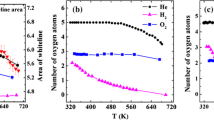
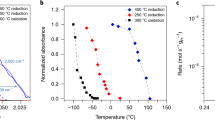
Abstract
The manifestations of strong and reactive metal–support interaction after reduction at elevated temperatures in Pt/V2O3 and Pd/V2O3 systems were studied using a dedicated thin film model system. Pt and Pd particles were prepared by electron-beam evaporation on NaCl(001) growth templates and subsequently embedded in a crystalline V2O3 matrix, prepared by thermal evaporation of V metal in 10−4 mbar oxygen pressure. Template temperatures of 600 K were used to induce the formation of epitaxially-ordered metal–oxide systems. Engineering of the metal–support interface by distinct annealing treatments allows steering the extent and quality of metal–support interaction. Whereas for Pt/V2O3 catalysts, high-temperature reduction at 773 K in hydrogen causes the epitaxial formation of a well-ordered body-centered tetragonal Pt3V intermetallic phase, the Pd/V2O3 system is mostly unaffected by similar treatments and remains in a metal–oxide state. Nevertheless, oxidation at 773 K of both catalysts prior to the hydrogen treatments lifts the epitaxial relation between metal and oxide and in turn, subsequent reduction at high-temperatures (T ≥ 773 K) yields only polycrystalline Pt3V and Pd3V intermetallic phases without particular ordering with respect to the former growth substrate. Along with this formation of intermetallic phases goes a transformation of the support stoichiometry from V2O3 to VO. Catalyst regeneration by partial oxidative decomposition of the intermetallic state is only possible at high-temperatures (T ≥ 750 K), yielding mostly metal particles and vanadium oxides with oxygen contents higher than V:O = 1:2, in particular V3O7.
Graphical Abstract
Adjusting the extent of metal–support contact area by annealing treatments allows for easy steering the structure and morphology of well-defined intermetallic compounds in Pt–VOx and Pd–VOx systems. Well-defined Pt3V intermetallic compounds are formed by direct reduction, whereby lifting the ordering by pre-oxidation yields less-defined compounds with altered metal–support contact area and consequently, strong metal–support interaction.










Similar content being viewed by others
References
van der Lee G, Schuller B, Post H, Favre TLF, Ponec V (1986) J Catal 98:522
van der Lee G, Bastein A, van den Boogert J, Schuller B, Luo HY, Ponec V (1987) J Chem Soc, Faraday Trans 83:2103
Ito S, Ishiguro S, Hagashima K, Kunimori K (1985) Catal Lett 55:197
Kowalski J, van der Lee G, Ponec V (1985) Appl Catal 19:423
Boffa AB, Bell AT, Somorjai GA (1993) J Catal 139:602
Beutel T, Knözinger H, Siborov AV, Zaikovskii VI (1992) J Chem Soc, Faraday Trans 88:2775
Sigl M, Brafrod M, Knözinger H, Vannice MA (1999) Top Catal 8:211
Kohl A, Linsmeier C, Taglauer E, Knözinger H (2001) PCCP 3:4639
Neyertz C, Volpe MA, Cigola C (2000) Catal Today 57:255
Haller GL, Resasco DE (1989) Adv Catal 36:173
Burch R (1988) In: Paal Z, Menon PG (eds) Hydrogen effects in catalysis, Marcel Dekker, Amsterdam p 347
Petukhov M, Rizzi GA, Granozzi G (2001) Thin Solid Films 406:154
Landolt Börnstein Phase equilibria of binary alloys, New Series III/7b1, Springer, Heidelberg ( 1975)
Surnev S, Sock M, Ramsey MG, Netzer FP, Klötzer B, Unterberger W, Hayek K (2002) Surf Sci 511:392
Hartmann T, Knözinger H (1996) Z Phys Chem 197:113
Krenn G, Schennach R (2004) J Chem Phys 120:5729
Ito S, Chibana C, Nagashima K, Kameoka S, Tomishige K, Kunimori K (2002) Appl Catal A 236:113
Reichl W, Hayek K (2004) J Catal 222:53
Reichl W, Hayek K (2002) J Catal 208:422
Ehrich H, Berndt H, Pohl MM, Jähnisch K, Baerns M (2002) Appl Catal A 230:271
Penner S, Wang D, Schlögl R, Hayek K (2005) Thin Solid Films 484:10
Penner S, Jenewein B, Wang D, Schlögl R, Hayek K (2006) Appl Catal A 308:31
Jenewein B, Penner S, Hayek K (2006) Appl Catal A 308:43
Penner S, Jenewein B, Wang D, Schlögl R, Hayek K (2006) PCCP 8:1223
Behrens M, Armbrüster M (2011) Methanol steam reforming, catalysis for alternative energy generation. Springer, New York, pp 175–235
Hucknall DJ (1974) Selective oxidation of hydrocarbons. Academic Press, London, p 212
Rupprechter G, Hayek K, Rendon L, Yacaman MJ (1995) Thin Solid Films 260:148
Powder Diffraction File, ICDD 1994, PDF Series 2 Sets 1-47, pattern 85-1403
Dwight DE, Downey JW, Conner RA (1961) Acta Cryst 14:75
Arbuzhov M (1981) Inorg Mater 17:300
Darriet J, Galy J (1972) J Solid State Chem 4:357
Penner S, Klötzer B, Jenewein B (2007) PCCP 9:2428
Koster W, Gmohling W (1960) Z Metallkd 51:385
Landolt Börnstein Phase equilibria of binary alloys, New Series IV/51, ch. 64, Springer, Heidelberg (1991)
Landolt Börnstein (1991) Phase equilibria of binary alloys, New Series IV/51, ch. 68, Springer, Heidelberg
Penner S, Wang D, Su DS, Rupprechter G, Podloucky R, Schlögl R, Hayek K (2003) Surf Sci 532–535:276
Wang D, Penner S, Su DS, Rupprechter G, Hayek K, Schlögl R (2003) J Catal 219:434
Shi AC, Masel RI (1989) J Catal 120:421
Harris PJF (1987) Surf Sci 185:L459
Ahmadi TS, Wang ZL, Green TC, Henglein A, El-Sayed MA (1996) Science 272:1924
Acknowledgments
We thank the Austrain Science foundation (FWF) for financial support under project F4503-N16, which is also performed within the framework of the Forschungsplattform Materials- and Nanoscience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penner, S., Stöger-Pollach, M. & Thalinger, R. Metal–Support Interaction in Pt/VOx and Pd/VOx Systems: A Comparative (HR)TEM Study. Catal Lett 144, 87–96 (2014). https://doi.org/10.1007/s10562-013-1095-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1095-2