Abstract
Purpose
Studies have shown that screen detection by national screening programs is independently associated with better prognosis of breast cancer. The aim of this study is to evaluate the association between tumor biology according to the 70-gene signature (70-GS) and survival of patients with screen-detected and interval breast cancers.
Methods
All Dutch breast cancer patients enrolled in the MINDACT trial (EORTC-10041/BIG3-04) accrued 2007–2011, who participated in the national screening program (biennial screening, ages 50–75) were included (n = 1102). Distant Metastasis-Free Interval (DMFI) was evaluated according to the 70-GS for patients with screen-detected (n = 754) and interval cancers (n = 348).
Results
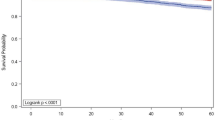
Patients with screen-detected cancers had 8-year DMFI rates of 98.2% for 70-GS ultralow-, 94.6% for low-, and 93.8% for high-risk tumors (p = 0.4). For interval cancers, there was a significantly lower 8-year DMFI rate for patients with 70-GS high-risk tumors (85.2%) compared to low- (92.2%) and ultralow-risk tumors (97.4%, p = 0.0023). Among patients with 70-GS high-risk tumors, a significant difference in 8-year DMFI rate was observed between interval (85.2%, n = 166) versus screen-detected cancers (93.8%, n = 238; p = 0.002) with a HR of 2.3 (95%CI 1.2–4.4, p = 0.010) adjusted for clinical-pathological characteristics and adjuvant systemic treatment.
Conclusion
Among patients with 70-GS high-risk tumors, a significant difference in DMFI was observed between screen-detected and interval cancers, suggesting that method of detection is an additional prognostic factor in this subgroup and should be taken into account when deciding on adjuvant treatment strategies.


Similar content being viewed by others
Data availability
The MINDACT dataset with patient characteristics and clinical outcomes was made available by the EORTC (https://www.eortc.org/data-sharing/). Following a successful data request procedure, the EORTC can share all or a selection of the clinical-pathological and/or full-transcriptome data for translational research.
References
Welch HG, Prorok PC, O’Malley AJ, Kramer BS (2016) Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 375:1438–1447. https://doi.org/10.1056/NEJMoa1600249
Nagtegaal ID, Allgood PC, Duffy SW et al (2011) Prognosis and pathology of screen-detected carcinomas. Cancer 117:1360–1368. https://doi.org/10.1002/cncr.25613
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England) 365:1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
Autier P, Boniol M, Koechlin A et al (2017) Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ 359:j5224. https://doi.org/10.1136/bmj.j5224
Integraal kankercentrum Nederland (2018) Borstkanker in Nederland—Trends 1989–2017. https://iknl.nl/getmedia/e2abd7e5-c6e5-4398-842f-e91facd3d6bd/iknl_rapport-borstkanker-in-nederland-311018-int.pdf
Mook S, Van’t Veer LJ, Rutgers EJ et al (2011) Independent prognostic value of screen detection in invasive breast cancer. JNCI J Natl Cancer Inst 103:585–597. https://doi.org/10.1093/jnci/djr043
Drukker CA, Schmidt MK, Rutgers EJT et al (2014) Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat 144:103–111. https://doi.org/10.1007/s10549-013-2830-5
Rayson D, Payne JI, Abdolell M et al (2011) Comparison of clinical-pathologic characteristics and outcomes of true interval and screen-detected invasive breast cancer among participants of a canadian breast screening program: a nested case-control study. Clin Breast Cancer 11:27–32. https://doi.org/10.3816/CBC.2011.n.005
Vitak B, Stål O, Månson JC et al (1997) Interval cancers and cancers in non-attenders in the ostergotland mammographic screening programme. Duration between screening and diagnosis, S-phase fraction and distant recurrence. Eur J Cancer Part A 33:1453–1460. https://doi.org/10.1016/S0959-8049(97)00185-8
Porter PL, El-Bastawissi AY, Mandelson MT et al (1999) Breast tumor characteristics as predictors of mammographic detection: Comparison of interval- and screen-detected cancers. J Natl Cancer Inst 91:2020–2028. https://doi.org/10.1093/jnci/91.23.2020
Esserman LJ, Thompson IM, Reid B et al (2014) Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 15:e234–e242. https://doi.org/10.1016/S1470-2045(13)70598-9
Azim HA, Michiels S, Zagouri F et al (2013) Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement†. Ann Oncol 24:647–654. https://doi.org/10.1093/annonc/mds645
van’t Veer LJ, Dai H, van de Vijver MJ et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536. https://doi.org/10.1038/415530a
van de Vijver MJ, He YD, van’t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009. https://doi.org/10.1056/NEJMoa021967
Buyse M, Loi S, van’t Veer L et al (2006) Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. JNCI J Natl Cancer Inst 98:1183–1192. https://doi.org/10.1093/jnci/djj329
Bueno-de-Mesquita JM, Linn SC, Keijzer R et al (2009) Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat 117:483–495. https://doi.org/10.1007/s10549-008-0191-2
Drukker CA, Bueno-De-Mesquita JM, Retèl VP et al (2013) A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer 133:929–936. https://doi.org/10.1002/ijc.28082
Cardoso F, van Veer LJ, Bogaerts J et al (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717–729. https://doi.org/10.1056/NEJMoa1602253
Piccart M, van’t Veer LJ, Poncet C et al (2021) 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol 22:476–488. https://doi.org/10.1016/S1470-2045(21)00007-3
Burstein HJ, Curigliano G, Loibl S et al (2019) Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 30:1541–1557. https://doi.org/10.1093/annonc/mdz235
Andre F, Ismaila N, Henry NL et al (2019) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update-integration of results from TAILORx. J Clin Oncol 37:1956–1964. https://doi.org/10.1200/JCO.19.00945
Cardoso F, Kyriakides S, Ohno S et al (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194–1220. https://doi.org/10.1093/annonc/mdz173
Delahaye LJMJ, Drukker CA, Dreezen C et al (2017) A breast cancer gene signature for indolent disease. Breast Cancer Res Treat 164:461–466. https://doi.org/10.1007/s10549-017-4262-0
Esserman LJ, Shieh Y, Rutgers EJT et al (2011) Impact of mammographic screening on the detection of good and poor prognosis breast cancers. Breast Cancer Res Treat 130:725–734. https://doi.org/10.1007/s10549-011-1748-z
Fracheboud J, de Koning HJ, Beemsterboer PMM et al (1998) Nation-wide breast cancer screening in The Netherlands: results of initial and subsequent screening 1990–1995. Int J Cancer 75:694–698. https://doi.org/10.1002/(SICI)1097-0215(19980302)75:5%3c694::AID-IJC6%3e3.0.CO;2-U
Li J, Ivansson E, Klevebring D et al (2017) Molecular differences between screen-detected and interval breast cancers are largely explained by PAM50 subtypes. Clin Cancer Res 23:2584–2592. https://doi.org/10.1158/1078-0432.CCR-16-0967
Cheasley D, Li N, Rowley SM et al (2019) Molecular comparison of interval and screen-detected breast cancers. J Pathol 248:243–252. https://doi.org/10.1002/path.5251
Wolf DM, Yau C, Sanil A et al (2017) DNA repair deficiency biomarkers and the 70-gene ultra-high risk signature as predictors of veliparib/carboplatin response in the I-SPY 2 breast cancer trial. NPJ Breast Cancer 3:1–8. https://doi.org/10.1038/s41523-017-0025-7
Kuijer A, Drukker CA, Elias SG et al (2016) Changes over time in the impact of gene-expression profiles on the administration of adjuvant chemotherapy in estrogen receptor positive early stage breast cancer patients: a Nationwide study. Int J Cancer 139:769–775. https://doi.org/10.1002/ijc.30132
Pérez Ramírez S, del Monte-Millán M, López-Tarruella S et al (2020) Prospective, multicenter study on the economic and clinical impact of gene-expression assays in early-stage breast cancer from a single region: the PREGECAM registry experience. Clin Transl Oncol 22:717–724. https://doi.org/10.1007/s12094-019-02176-x
Retèl VP, Byng D, Linn SC et al (2020) Cost-effectiveness analysis of the 70-gene signature compared with clinical assessment in breast cancer based on a randomised controlled trial. Eur J Cancer 137:193–203. https://doi.org/10.1016/j.ejca.2020.07.002
Wishart GC, Azzato EM, Greenberg DC et al (2010) PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 12:R1. https://doi.org/10.1186/bcr2464
Acknowledgements
We acknowledge the contribution of the European Organisation for Research and Treatment of Cancer (EORTC) and the Breast International Group (BIG). We thank the Dutch Screening Facilities for providing the screening data used in this study. We thank Annuska Glas from Agendia for providing valuable information on the 70-gene signature results. We are indebted to all the Dutch women who participated in the MINDACT trial. Josephine Lopes Cardozo’s work as Fellow at EORTC Headquarters was supported by a grant from the EORTC Breast Cancer Group and from the Netherlands Cancer Institute. The funding sources had no role in the study design, data collection, data analysis, data interpretation, in writing the report, or in the decision to submit for publication. The MINDACT study was supported by grants from the European Commission Sixth Framework Program (FP6-LSHC-CT-2004-503426, to the TRANSBIG Network of Excellence), the Breast Cancer Research Foundation, Novartis, F. Hoffmann–La Roche, Sanofi-Aventis, Eli Lilly, Veridex, the European Breast Cancer Council–Breast Cancer Working Group (BCWG grant for the MINDACT biobank), the Jacqueline Seroussi Memorial Foundation for Cancer Research (JSMF; 2006 JSMF Award), Prix Mois du Cancer du Sein (2004 award), Susan G. Komen for the Cure (SG05-0922-02), Fondation Belge contre le Cancer (SCIE 2005-27), Dutch Cancer Society (KWF), the Netherlands Genomics Initiative–Cancer Genomics Center (2008-2012), Association le Cancer du Sein, Parlons-en!, the Brussels Breast Cancer Walk-Run and the American Women’s Club of Brussels, NIF Trust, German Cancer Aid, the Grant Simpson Trust and Cancer Research UK, Ligue Nationale contre le Cancer, and the EORTC Cancer Research Fund. Whole-genome analysis was provided by Agendia without cost. We are grateful to all patients and families who participated in the MINDACT study. We are grateful to the European Commission Sixth Framework Programme (FP6-LSHC-CT-2004-503426), the European Community Seventh Framework Programme (HEALTH-F2-2009-223175 to the Collaborative Oncological Gene-environment Study), the Breast International Group (BIG) AISBL, F. Hoffmann-La Roche, Novartis, Sanofi-Aventis, for supporting this independent EORTC Study. Special thanks to all national coordinating centers and BIG Groups participating in MINDACT (BOOG, EORTC-BCG, GOIRC, NCRI-BCSG, SOLTI, UNICANCER-UCBG, WSG).
Funding
This research was supported by a grant from the EORTC Breast Cancer Group and from the Netherlands Cancer Institute. The MINDACT trial was supported by grants from the European Commission Sixth Framework Program (FP6-LSHC-CT-2004–503426, to the TRANSBIG Network of Excellence), the Breast Cancer Research Foundation, Novartis, F. Hoffmann–La Roche, Sanofi-Aventis, Eli Lilly, Veridex, the European Breast Cancer Council–Breast Cancer Working Group (BCWG grant for the MINDACT biobank), the Jacqueline Seroussi Memorial Foundation for Cancer Research (JSMF; 2006 JSMF Award), Prix Mois du Cancer du Sein (2004 award), Susan G. Komen for the Cure (SG05-0922-02), Fondation Belge contre le Cancer (SCIE 2005-27), Dutch Cancer Society (KWF), the Netherlands Genomics Initiative–Cancer Genomics Center (2008–2012), Association le Cancer du Sein, Parlons-en!, the Brussels Breast Cancer Walk-Run and the American Women’s Club of Brussels, NIF Trust, German Cancer Aid, the Grant Simpson Trust and Cancer Research UK, Ligue Nationale contre le Cancer, and the EORTC Cancer Research Fund. Whole-genome analysis was provided by Agendia without cost.
Author information
Authors and Affiliations
Contributions
CD, MKS, LvtV, ER, and FC were responsible for the study design and development of the protocol. JLC and CD coordinated the study and CD collected the data on method of detection. FC and CP provided the MINDACT baseline characteristics and outcome data. JLC, CD, and MKS performed the data analysis. All authors took part in data interpretation. The first draft of the manuscript was written by JLC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
L.J. van ‘t Veer reports being shareholder in and part-time employed by Agendia NV, the commercial company that markets the 70-gene signature as MammaPrint. F. Cardoso has received personal fees from Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Samsung Bioepis, Sanofi, Seagen, and Teva outside the submitted work.
Ethical approval
This study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The MINDACT study was approved by the ethics commitees of all participating sites. This project was approved by the MINDACT Executive Committee and MINDACT Steering Committee, after review by experts from the MINDACT Independent Review Committee, the MINDACT Statistician, and the MINDACT Ethical-Legal committee. This project was reviewed by the ethics committee who concluded it was a non-CT directive study that did not require further ethical approval, and could be performed under the scope of the MINDACT protocol and informed consent.
Consent to participate
All patients included in the MINDACT trial provided written informed consent. This consent allowed linkage to the Dutch national screening database.
Consent for publication
All authors reviewed draft and final versions of the manuscript before submission and approved the final version. The first and last authors had full access to all of the data and had final responsibility for the decision to submit for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lopes Cardozo, J.M.N., Schmidt, M.K., van ’t Veer, L.J. et al. Combining method of detection and 70-gene signature for enhanced prognostication of breast cancer. Breast Cancer Res Treat 189, 399–410 (2021). https://doi.org/10.1007/s10549-021-06315-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06315-3