Abstract
Purpose
Immune checkpoints cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death 1 receptor (PD-1) negatively regulate CD8+ T cell functions, impeding the capacity of effector T cells to kill tumors. Here, we study the prognostic significance of CTLA4, PD-1 and T cell activation status in breast cancer.
Methods
Using a publicly accessed RNA-seq dataset including 1087 breast cancer patients, we performed Kaplan–Meier survival curves and multivariate Cox regression models to evaluate the associations of CTLA4, PD-1, and weighted T cell activation score with patients’ overall survival.
Results
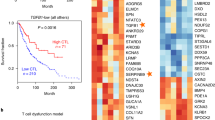
Survival analyses showed that high CTLA4 but low PD-1 expression was associated with a poor overall survival, and that high T cell activation score was associated with an improved survival. The median survival was 216.6 months (95% CI 114.1–244.9) for the T activation group, 127.0 months (95% CI 112.3–212.1) for the intermediate, and 120.5 months (95% CI 93.8 to ∞) for the exhaustion (Log-rank p = 0.084). This association was verified in multivariate Cox regression analysis. The hazard ratios were 0.81 (95% CI 0.56–1.19) for the intermediate group, and 0.48 (95% CI 0.26–0.86) for the activation group, respectively, in comparison to the exhaustion group (p value for trend = 0.016).
Conclusions
T cell activation score has significantly positive relationship with patients’ overall survival, and may serve as a marker of personalized immunotherapy in breast cancer patients. Cocktail rather than single immune checkpoint blockade may yield more benefit for breast cancer patients.

Similar content being viewed by others
References
Chen L, Han X (2015) Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 125(9):3384–3391. doi:10.1172/JCI80011
Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, Donia M, Boschen ML, Lund-Johansen F, Olweus J, Schumacher TN (2016) Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 352(6291):1337–1341. doi:10.1126/science.aaf2288
Kim T, Amaria RN, Spencer C, Reuben A, Cooper ZA, Wargo JA (2014) Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol Med 11(4):237–246. doi:10.7497/j.issn.2095-3941.2014.04.002
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. doi:10.1056/NEJMoa1003466
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364(26):2517–2526. doi:10.1056/NEJMoa1104621
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. doi:10.1056/NEJMoa1412082
Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16(4):375–384. doi:10.1016/S1470-2045(15)70076-8
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, Cranmer LD, Blank CU, O’Day SJ, Ascierto PA, Salama AK, Margolin KA, Loquai C, Eigentler TK, Gangadhar TC, Carlino MS, Agarwala SS, Moschos SJ, Sosman JA, Goldinger SM, Shapira-Frommer R, Gonzalez R, Kirkwood JM, Wolchok JD, Eggermont A, Li XN, Zhou W, Zernhelt AM, Lis J, Ebbinghaus S, Kang SP, Daud A (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16(8):908–918. doi:10.1016/S1470-2045(15)00083-2
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 34(21):2460–2467. doi:10.1200/JCO.2015.64.8931
Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, Lanoy E, Texier M, Libenciuc C, Eggermont AM, Soria JC, Mateus C, Robert C (2016) Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 13(8):473–486. doi:10.1038/nrclinonc.2016.58
Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J (2015) Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer Immunol Immunother 64(7):853–860. doi:10.1007/s00262-015-1696-2
Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang J (2016) Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol 18(5):497–506. doi:10.1007/s12094-015-1391-y
Sun S, Fei X, Mao Y, Wang X, Garfield DH, Huang O, Wang J, Yuan F, Sun L, Yu Q, Jin X, Wang J, Shen K (2014) PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother 63(4):395–406. doi:10.1007/s00262-014-1519-x
Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE (2013) The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 139(3):667–676. doi:10.1007/s10549-013-2581-3
Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL (2014) In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 20(10):2773–2782. doi:10.1158/1078-0432.CCR-13-2702
Guo Y, Yu P, Liu Z, Maimaiti Y, Wang S, Yin X, Liu C, Huang T (2016) Prognostic and clinicopathological value of programmed death ligand-1 in breast cancer: a meta-analysis. PLoS ONE 11(5):e0156323. doi:10.1371/journal.pone.0156323
Tirosh I, Izar B, Prakadan SM, Wadsworth MH 2nd, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jane-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA (2016) Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352(6282):189–196. doi:10.1126/science.aad0501
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. doi:10.1038/nature11412
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):pl1. doi:10.1126/scisignal.2004088
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517):202–209. doi:10.1038/nature13480
Contal C, O’Quigley J (1999) An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal 30:253–270
Mandrekar JN, Mandrekar S, Cha SS (2006) Cutpoint determination methods in survival analysis using SAS®. In: Proc 28th SAS users group international conference. pp 261–228. http://www2.sas.com/proceeding/sugi27/261-28.pdf
Lu L, Katsaros D, Canuto EM, Biglia N, Risch HA, Yu H (2016) LIN-28B/let-7a/IGF-II axis molecular subtypes are associated with epithelial ovarian cancer prognosis. Gynecol Oncol 141(1):121–127. doi:10.1016/j.ygyno.2015.12.035
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548. doi:10.1146/annurev.immunol.23.021704.115611
Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman GJ, Krogsgaard M, Riley JL (2013) Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA 110(27):E2480–E2489. doi:10.1073/pnas.1305394110
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi:10.1146/annurev.immunol.26.021607.090331
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499. doi:10.1038/nri3862
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. doi:10.1038/nrc3239
Chen L, Flies DB (2013) Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13(4):227–242. doi:10.1038/nri3405
Ott PA, Hodi FS, Robert C (2013) CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 19(19):5300–5309. doi:10.1158/1078-0432.CCR-13-0143
Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC (2005) Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 11(2 Pt 1):728–734
Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM (2012) CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res 72(4):887–896. doi:10.1158/0008-5472.CAN-11-2637
Wherry EJ (2011) T cell exhaustion. Nat Immunol 12(6):492–499
Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK (2011) Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol 32(8):345–349. doi:10.1016/j.it.2011.05.003
Goldberg MV, Drake CG (2011) LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol 344:269–278. doi:10.1007/82_2010_114
Bense RD, Sotiriou C, Piccart-Gebhart MJ, Haanen JB, van Vugt MA, de Vries EG, Schroder CP, Fehrmann RS (2017) Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst 109(1):djw192. doi:10.1093/jnci/djw192
Acknowledgements
We thank Dr. Liqiong Gui at Yale School of Medicine for her critical comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflict of interests to declare.
Ethical approval
All procedures performed in this study involving human subjects followed the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. The study presented here complies with the current laws of the United States of America.
Rights and permissions
About this article
Cite this article
Lu, L., Bai, Y. & Wang, Z. Elevated T cell activation score is associated with improved survival of breast cancer. Breast Cancer Res Treat 164, 689–696 (2017). https://doi.org/10.1007/s10549-017-4281-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4281-x