Abstract
Purpose
The data of 589 metastatic breast cancer (MBC) patients in a single institution were reviewed to determine the outcomes of patients with brain metastasis (BM) and assess the efficacy of BM screening.
Methods
The patients with BM among the 589 MBC patients who underwent treatment at Shizuoka Cancer Center (Shizuoka, Japan) from 09/2002 to 03/2014 were retrospectively analyzed.
Results
During the study period, BM developed in 187 (31.7%) patients. The tumor subtypes were as follows: luminal (hormone receptor [HR]+, HER2−), 44.9%; luminal-HER2 (HR+, HER2+), 14.9%; HER2 (HR−, HER2+), 21.3%; and triple-negative (TN), 16.0%. BM was detected in 48.6% of the patients by screening MRI. While 137 of 187 patients underwent local therapy, whole-brain irradiation was the most frequently applied therapy (63.5%). The median overall survival from the diagnosis of BM was as follows: luminal, 7.0 months (M); luminal-HER2, 13.3 M; HER2, 17.7 M; TN, 4.2 M. The HER2 status (hazard ratio [HR]: 0.58, 95% confidence interval [CI] 0.38–0.88) and nonprogressive extracranial lesion(s) (HR: 0.45, 95% CI 0.29–0.71) were identified as prognostic factors in a multivariate analysis. When limited to HER2-overexpressed MBC patients, the multivariate analysis revealed that non-progressive extracranial lesion(s) (HR: 0.20, 95% CI 0.088–0.47) and stereotactic irradiation (STI) as an initial treatment (HR: 0.18, 95% CI 0.061–0.56) were prognostic factors.
Conclusions
Our retrospective review showed that early detection of BM by screening MRI, followed by STI, improved the prognosis of HER2-overexpressed MBC patients with BM. A further prospective randomized study is needed to confirm our findings.
Similar content being viewed by others
Introduction
The incidence of brain metastasis (BM) in stage I/II early breast cancer (EBC) is ≤2% at 5 years following breast surgery and ≤5% at 10 years [1]. However, 5–15% of patients with metastatic breast cancer (MBC) are known to develop BM during their clinical course [2, 3], and due to recent improvements in diagnostic imaging and increased life expectancy due to developments in systemic treatment, the incidence of BM in MBC patients is considered to have increased to >30% [4, 5]. The risk of BM differs depending on the subtype of breast cancer, with an increased tendency for complications in those with human epidermal growth factor receptor type 2 (HER2) overexpression or triple negative (TN) [3, 4, 6–8]. The incidence of BM in HER2-overexpressed subtype in particular (i.e. HER2-positive MBC [HER2+MBC]) has been reported to be 30–50%. [4, 9–12].
However, while the frequency of BM in MBC is relatively high, it is currently believed that prospective screening for BM does not contribute to the prognosis [3, 13]; thus, the ASCO guidelines do not recommend routine screening for HER2+MBC patients [9]. Instead, physicians are urged to be alert for suspicious clinical symptoms. However, with recent developments in drug and radiation therapies, early detection of BMs (i.e., diagnosing and treating an asymptomatic patient identified by screening) may contribute to improved prognoses. We examined the usability of BM screening by retrospectively analyzing the 12-year follow-up data at our hospital and evaluated the necessity of a randomized controlled trial (RCT).
Methods
We retrospectively analyzed the patients with BM among the 589 MBC patients who underwent treatment at the Shizuoka Cancer Center (Shizuoka, Japan) from September 2002 to March 2014. The backgrounds and therapeutic courses of patients were collected through electronic medical charts.
Regarding subtypes, the subtypes at the time of surgery or at the first biopsy were generally used; however, the biopsy results at recurrence were used when available. Subtype classification was carried out based on estrogen receptor (ER), progesterone receptor (PR), and HER2; ≥1% of cells being positive for ER and/or PR was defined as ‘hormone receptor (HR) positive,’ while HER2-overexpression was determined according to the 2007 ASCO/CAP guidelines [14]. Based on the results of HR and HER2, each subgroup was determined in this study as follows: luminal (HR+, HER2−), luminal-HER2 (HR+, HER2+), HER2 (HR−, HER2+), and triple negative (TN).
For the survival analysis, the following were assessed by a multivariate analysis as factors affecting the overall survival (OS) from the MBC diagnosis and OS from the BM diagnosis (BMOS): the age at BM diagnosis; subtype; disease-free interval (DFI) from EBC or MBC diagnosis to BM; trigger of BM diagnosis (without symptom [i.e. by screening] or due to any symptom); Karnofsky performance status (KPS) at BM diagnosis; metastatic site(s) at BM diagnosis; controlling status of non-BM lesions at BM diagnosis judged by clinically and/or radiographically; number of BM lesion(s); image of BM (solid or cystic); presence of leptomeningeal disease (LMD); local treatment for BM (radiotherapy, surgery); and history of lapatinib use. Once a patient was diagnosed with BM, treatment for BM was provided in cooperation with a neurosurgeon and/or a radiologist.
The Kaplan–Meier method was used for the survival analysis, and a log-rank test was used for comparisons among groups, with the significant difference set at p < 0.05. Regarding the analysis of prognosis factors, univariate and multivariate analyses using the Cox proportional hazards model were used. The Excel Statistics version 1.13 (Social Survey Research Information Co., Ltd., Tokyo, Japan) software program was used as the statistical software.
Results
Patient background
Of the 589 MBC patients who underwent treatment at our hospital during the period from September 2002 through March 2014, BM was observed in 187 patients (31.7%), with 28 (4.7%) already complicated with BM at MBC diagnosis. In five patients (0.8%), BM was the only metastatic site of their initial MBC diagnosis. BM was commonly observed as a complication of HER2 + MBC patients (luminal-HER2 52.8%, HER2+ 46.5%). LMD was observed in 37 patients (6.2%), with 23 of the 187 BM patients (12.2%) observed therewith at the time of BM diagnosis (median duration of follow-up: 30.8 months [range: 0–218.1]).
The background of patients with BM is shown in Table 1 (five patients were not fully documented). The breakdown of subtypes was luminal in 84 patients (44.9%), luminal-HER2 in 28 patients (14.9%), HER2+ in 40 patients (21.3%), TN in 30 patients (16.0%), with a median time to BM from MBC of 20.0, 27.9, 14.9, and 17.7 months, respectively. Poor control of extracranial lesion(s) judged by clinically and/or radiographically at the time of BM diagnosis was observed in 64.2% of luminal, in 41.1% of HER2+, and in 70.0% of TN patients.
A total of 95 patients (50.8%) exhibited symptoms at the time of BM, while 91 (48.6%) were diagnosed with BM at screening (i.e. asymptomatically), excluding 1 patient whose symptoms were not fully documented, and BM tended to be detected with symptoms more often in TN patients than in other subtypes (19 of 30 patients; 63.3%). There was no significant difference in the number of BM lesion(s) among patients, regardless of their subtype. While most of the BMs were solid in form, cystic forms were observed in 15 patients (8.0%).
Treatment
Of the 187 patients diagnosed with BM, 137 patients (73.2%) required immediate local treatment (Fig. 1). The initiation of treatment was deferred in the other 50 patients for reasons such as showing extremely poor general status or BM was minimal. Eventually, 148 patients (79.1%) underwent treatment for BM. Whole-brain irradiation (WBI) was selected as the initial therapy for 94 patients (63.5%). Surgery was performed as the initial therapy in 12 patients (8.1%), with WBI added postoperatively in each case. WBI was added to stereotactic irradiation (STI) in one patient only, while STI alone was conducted in 37 patients (25.0%). Of the 148 patients who underwent local therapy, 47 (31.7%) were diagnosed with recurrence of BM, with 23 of 94 patients (24.4%) treated by WBI and 15 of 37 patients (40.5%) treated by STI, wherein the median time to subsequent STI was 38.2 months and 27.5 months, respectively. The median number of sessions of additional therapy with STI was 1 (range: 1–5). Multimodal therapy for LMD, such as intrathecal injections of methotrexate or WBI combined with whole-spine irradiation (WSI), was administered to five (luminal, n = 4; TN, n = 1) patients.
Treatments for brain metastasis. Of the 187 patients diagnosed with BM, 137 (73.2%) required immediate local treatment. The initiation of treatment was deferred in the other 50 patients. Eventually, treatment for BM was introduced in 148 patients (79.1%). WBI was selected as the initial therapy for 94 patients (63.5%). Surgery was performed as the initial therapy in 12 patients (8.1%), with WBI added postoperatively in each case. WBI was added to STI in one patient, while STI alone was performed in 37 patients (25.0%)
Outcome
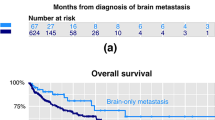
At the data cutoff date, 169 patients (89.4%) have died. The causes of death were as follows: BM-related, n = 49 (28.9%); respiratory failure, n = 49 (28.9%); hepatic failure, n = 25 (14.7%); cachexia, n = 36 (21.3%); infection, n = 5 (2.9%); and “other,” n = 5 (2.9%). Regarding the prognosis from MBC onset, the median survival was 45.8 months (95% CI 42.7–51.2) among MBC patients overall, 53.4 months (95% CI 47.6–63.1) in MBC without BM, and 37.7 months (95% CI 30.2–42.7) in MBC with BM (Fig. 2). In the TN subtype, the presence/absence of BM did not affect the OS (OS from MBC: BM+, 27.9 months [95% CI 17.8–35.3]; BM−, 33.1 months [95% CI 23.6–36.3]; p = 0.35, log-rank) (Fig. 3).
The median BMOS (mBMOS) among patients overall was 9.7 months (95% CI 7.3–11.5), and the mBMOS by subtypes was as follows: luminal, 7.0 months; luminal-HER2, 13.3 months; HER2, 17.7 months; and TN, 4.2 months, all of which differed significantly (p < 0.01, log-rank) (Fig. 4). The BMOS of HER2-overexpressed patients, i.e. luminal-HER2 and HER2, was significantly longer than that of HER2-negative patients (hazard ratio [HR] 0.47 [95% CI 0.34–0.65] p < 0.001). The prognosis from LMD was extremely poor, at 4.0 months of median survival from the event (95% CI 1.8–5.6) with a high rate of LMD-related death (59.4%). In addition, patients with cystic lesions also demonstrated significantly poor prognosis (p = 0.016), with a mBMOS of 6.2 months (95% CI 2.1–8.1). On comparison based on the control status of extracranial lesion(s) judged by clinically and/or radiographically at the time of BM diagnosis, BMOS was significantly longer in groups in which the extracranial lesion(s) was not progressive (HR 0.48 [95% CI 0.32–0.70] p = 0.0002) than in the groups with progressive extracranial lesions.
The prognostic factors in the univariate analysis also included symptoms at the time of BM diagnosis (HR 1.48 ([95% CI 1.09–2.01] p = 0.011), STI performed as an initial therapy (HR 0.55 [95% CI 0.37–0.81] p = 0.0029), ≥4 BMs (HR 1.54 [95% CI 1.08–2.2] p = 0.017), and KPS at the time of BM diagnosis <70 (HR 1.87 [95% CI 1.35–2.57] p = 0.0001), while age (60 years or older) was not included. In the multivariate analysis, HER2-overexpression (HR 0.58 [95% CI 0.38–0.88] p = 0.012) and non-progressive extracranial lesions (HR 0.45 [95% CI 0.29–0.71] p = 0.0007) were selected as the prognosis factors (Table 2).
Subgroup analysis of HER2+MBC patients
In the analysis of BM patients overall, HER2+MBC patients were associated with a high frequency of BM, while the outcomes were better than in HER2−MBC patients; therefore, we analyzed the patient backgrounds and prognostic factors of HER2+MBC patients with BM (HER2+BM).
BM was detected at screening in 37 of the 139 HER2+MBC patients (26.6%). The background characteristics based on the presence/absence of symptoms at the time of BM development are shown in Table 3. The KPS was favorable in the screening group (KPS ≥ 70: screening group, 70.2%; symptomatic group, 20.0%), with a higher rate of patients with ≤4 BMs at the time of the diagnosis and a higher rate of patients eligible for STI in comparison to the symptomatic group (≤4 BMs: screening group, 72.9%; symptomatic group, 43.3% [p = 0.013, χ 2]; STI performed: screening group, 35.1%; symptomatic group, 10.0% [p = 0.016, χ 2]). The drugs used for systemic therapy in patients with BM were as follows: trastuzumab was administered to all patients; lapatinib, n = 27 (39.7%), pertuzumab, n = 0 (0%); and trastuzumab emtansine (T-DM1), n = 3 (4.4%).
The control status of extracranial lesions and the performance of STI significantly improved the outcomes (extracranial lesions: HR 0.42 [95% CI 0.23–0.79] p = 0.0075, STI performance: HR 0.44 [95% CI 0.23–0.84] p = 0.013). Furthermore, the outcomes were also improved by the use of a tyrosine kinase inhibitor (TKI), such as lapatinib (HR 0.56 [95% CI 0.32–0.96] p = 0.037). The number of BMs (HR 2.12 [95% CI 1.13–3.97] p = 0.018) and the KPS at the time of BM onset (HR 2.00 [95% CI 1.15–3.49] p = 0.013) were also identified as prognostic factors in the univariate analysis. For the multivariate analysis, stable or better control in other lesions (HR 0.20 [95% CI 0.088–0.47] p = 0.0002) and the performance of STI (HR 0.18 [95% CI 0.061–0.56] p = 0.0031) were identified as prognostic factors (Table 4).
Discussion
In breast cancer patients, as a result of improved outcomes due to advances in systemic therapy along with improvements in imaging modalities, the discovery rates of BM have increased [3–5, 9], with BM observed in 31.7% of overall MBC patients and 48.9% of HER2+MBC patients based on the analysis at our hospital. Many reports exist regarding the prognosis from the onset of BM, and according to the report by Niikura et al. [16] analyzing 1256 BM patients in Japan, the onset of BM within 6 months of MBC, an asymptomatic condition, HER2-overexpression, and HR positivity were selected as good prognostic factors for BMOS. In addition, the KPS at the time of BM onset, age, control status of extracranial lesions, number of BMs, and surgery/STI history have also been reported as prognostic factors [4, 17–19].
Sperduto et al. [15] proposed the diagnosis-specific graded prognostic assessment for breast cancer patients with BM (Breast-GPA), which scores the KPS at the time of BM diagnosis, subtype, and age, for the classification of patients into groups of 1 (worst)–4 (best) to infer the outcomes. While the median BMOS was 3.4 months in Group 1 (the worst prognosis), the median BMOS was 25.3 months in Group 4 (the best prognosis), indicating a considerable difference between the two. Similarly, in the evaluation at our hospital, we also observed a significant difference in the median BMOS among the Breast-GPA grades (Group 1: 4.1 months [1.5–5.3], Group 2: 7.0 months [5.0–9.7], Group 3: 13.0 months [8.9–16.9], and Group 4: 41.9 months [13.3-still alive], p < 0.00001). In contrast, no significant difference in BMOS was observed by age, which is one of the scores, in a univariate analysis.
The univariate analysis in the present study identified HER2-overexpression, stable or better extracranial lesions, STI as an initial therapy for BM, ≤4 BMs, and good KPS (70–100) at BM onset as good prognostic factors, and the multivariate analysis further identified HER2-overexpression (HR 0.58 [95% CI 0.38–0.88] p = 0.012) and stable or better extracranial lesions (HR 0.45 [95% CI 0.29–0.71] p = 0.0007) as good prognostic factors. In addition to the control of extracranial lesions by systemic therapy, in HER2+MBC patients, an improvement in prognosis can be expected by multimodal therapy for BM.
Leptomeningeal disease is known to be a complication in 5% of breast cancer cases overall [9, 20], and has a poor prognosis, with a median OS of 2.6–4.5 months [20–23]. Meningeal infiltration from BM, bone metastasis, lobular carcinoma, and TN are known risks for LMD [21–23]. Treatment for LMD includes WBI with/without WSI, systemic therapy, and intrathecal injection, all of which lack efficacy. At our hospital, LMD was observed in 6.2% of MBC patients overall and was derived from lobular carcinomas (including those exhibiting lobular carcinoma formations) in 13.5% of cases, with bone metastases observed as a complication in 72.9%. The median survival in LMD patients is as short as 4.0 months, with 59.4% having central nerve lesions as the cause of death, indicating a poor prognosis according to our medical records.
Median BMOS for HER2+BM is reported as 8.9–23.1 months [17–19, 24, 25], which is better than with other subtypes, and the results at our hospital also showed good prognoses of 13.3 months for Luminal-HER2 and 17.7 months for HER2. As number of novel agents are being developed for the treatments of HER2-overexpressed breast cancer, such as lapatinib, pertuzumab, and T-DM1 in addition to trastuzumab, further improvement in the outcome is expected. Of these, lapatinib is a TKI against HER1 and HER2 [26], and a study comparing lapatinib + capecitabine with capecitabine monotherapy found that complications of BMs were less frequent in the lapatinib + capecitabine cohort than in the capecitabine monotherapy cohort [27]. Furthermore, in a phase II study [28] in which lapatinib + capecitabine was used without local treatment for the brain in HER2+BM, the results were favorable, with an overall response rate of 65.9 and 20% of the cases demonstrating a decrease in tumor size of more than 80%. The time until requiring local treatment for the brain due to worsening of the disease state is a median of 8.3 months, and it was reported that the use of lapatinib + capecitabine can delay the initiation of WBI, which carries a risk of inducing a decline in the cognitive function [28]. In addition, Hayashi et al. [24], reported that patients with a treatment history for both lapatinib and trastuzumab had a better BMOS than others with no such history. The CLEOPATRA study found that although there was no marked difference in the BM complication rate, the time for the development of BM as a complication was delayed and the BMOS improved in the pertuzumab group in comparison to the control group [29]. Furthermore, reports exist on the BM control effect [30] and the treatment effect of trastuzumab emtansine [31]. Taken together, these previous findings suggest that the role of systemic drug therapy for BMs is changing.
The choice of local treatment for BMs includes surgery, WBI, and STI, with indications outlined in the guidelines by ASTRO2012 and ASCO for HER2-overexpression [9, 32]. Conventionally, WBI is the standard therapy for BM, and prophylactic WBI was once evaluated in HER2+MBC patients, where BMs are frequently observed [13, 33]. However, since the prognosis following BM complication has significantly improved with advancements in technology and treatments, concerns have risen regarding the risk of a decline in the cognitive function as a late-stage complication of WBI [34–37]. Although the recurrence rate following STI monotherapy for a small number of BMs is higher than that following WBI [37], favorable local control has been achieved with STI monotherapy [35, 38–40], and recently, STI monotherapy has been reported effective in managing 5–10 BMs [41]. At our hospital, as an initial treatment for BMs, STI was selected in 19.7% of cases; although no significant difference in the prognosis between STI and WBI was observed in a multivariate analysis, STI demonstrated better prognoses than other treatments in a univariate analysis (HR 0.55 [95% CI 0.37–0.81] p = 0.0029). The rate of requiring additional treatment was 40%, higher than the 24% required for WBI; however, the median time from the initial treatment to additional treatment was 27.5 months, demonstrating long-term disease control. For HER2+BM, performing STI was a favorable prognostic factor in a multivariate analysis (HR 0.18 [95% CI 0.061–0.569] p = 0.0031); in some cases, STI was carried out 5 times in total, controlling BM for 56.5 months. Since HER2-overexpression is expected to further improve the prognosis due to developments in systemic therapy, we believe that detecting BMs early and initiating STI treatment can delay the time until WBI, which carries a risk of reducing the cognitive function decline, and thereby maintain the quality of life.
In order to detect BMs at an early stage, we believe carrying out screening is preferable; however, in the current guidelines for HER2+MBC by ASCO [9], it is advised that screening not be routinely carried out, but rather any signs and symptoms not be overlooked. In a meta-analysis of clinical studies on prospective screening for brain metastatic breast cancer [3], no difference was observed in the prognoses. Furthermore, in a prospective study on BM screening in HER2+MBC patients [13], it was reported that although deaths related to the central nervous system were less frequent in the screening group, there was no marked difference between the groups in the prognosis overall. However, at the time these studies were carried out, the variations in the anti-HER2 treatments and the accuracy of MRI were different from the situation today; as such, these studies need to be interpreted with careful consideration.
We believe that the subjects for BM screening should be limited to those subtypes in which the frequency of BM is high and symptoms other than BM are able to be controlled (i.e. subtypes which have many choices for systemic drug therapies). Since luminal-type breast cancer has a relatively low rate of BM as a complication compared to other subtypes, luminal and TN breast cancer tend to exhibit poor control over extracranial lesions by systemic therapy when complicated by BMs, as shown in our study (worsening extracranial lesion[s] observed in 64.2% of luminal, 41.1% of HER2, and 70.0% of TN patients). However, TN has a poor prognosis regardless of BM as a complication, limiting the screening benefits of luminal and TN subtypes. In contrast, screening benefits can be expected in the HER2-overexpressed subtypes. In other words, while cases are frequently complicated by BM in general, extracranial lesions are relatively well-controlled even with BM, and in the evaluation at our institution, the rate of having ≤4 BMs at the time of diagnosis was relatively high (i.e. many patients were eligible for STI in the screening group), and the HR for carrying out STI was 0.18 (95% CI 0.061–0.56) in a multivariate analysis. Consequently, it is expected that, by detecting BM at an early stage through screening, the proportion of patients eligible for STI may increase, leading to further improvements in prognoses.
Unlike prospective randomized comparative trials, the present study had some limitations, such as its retrospective nature, the limited number of cases, and the lack of prospective randomization for screening. However, at the same time, the strengths of the study were its provision of a realistic picture based on actual clinical practice, the successful proposition of a clinical question regarding the need for prospective screening, and the lack of fluctuation between diagnosis and treatment, as it was a single-site study.
Conclusion
Our retrospective, single-institution review showed that metastasis to the brain is a major complication in metastatic breast cancer patients, regardless of their subtypes. However, when limited to HER2+MBC patients, brain metastasis is not always a terminal event; the systemic status can be improved in these patients, in contrast to luminal or triple-negative MBC developing brain metastasis. Furthermore, HER2+MBC patients can expect a better prognosis in the future by developments in target molecular treatments. Since early detection of BM leads to an increase in the proportion of patients eligible for effective treatment, screening brain MRI, not only at the time of MBC diagnosis but also at certain time intervals, may contribute to the patients with HER2 + MBC, and further verification of the prospective brain screening through an RCT is warranted.
References
Arvold ND, Oh KS, Niemieko A et al (2012) Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat 136:153–160
Barnholtz-Sloan JS, Solan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance system. J Clin Oncol 22:2865–2872
Miller KD, Weathers T, Haney LG et al (2003) Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol 14:1072–1077
Leyland-Jones B (2009) Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol 27:5278–5286
Lin NU, Jennifer RB, Eric PW (2004) CNS metastases in breast cancer. J Clin Oncol 22:3608–3617
Gabos Z, Sinha R, Hanson J et al (2006) Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 24:5658–5663
Kennecke H, Yerushalmi R, Woods R et al (2010) Metastatic behavior on breast cancer subtypes. J Clin Oncol 28:3271–3277
Lin NU, Claus E, Sohl J et al (2008) Sites of distant recurrence and clinical outcome in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113:2638–2645
Ramakrishna N, Temin S, Chandarlapaty S et al (2014) Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: american society of clinical oncology clinical practice guideline. J Clin Oncol 32:2001–2008
Gori S, Rimondini S, Anjelis VD et al (2007) Central nervous system metastases in HER-2-positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist 12:766–773
Bendell JC, Domchek SM, Burstein HJ et al (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast cancer. Cancer 97:2972–2977
Brufsky AM, Mayer M, Rugo HS et al (2011) Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834–4843
Niwinska A, Tacikowska M, Murawska M (2010) The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys 77:1134–1139
Wolff AC, Hammond ME, Hicks DG et al (2007) American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Sperduto PW, Kased N, Roberge D et al (2012) Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys 82:2111–2117
Niikura N, Hayashi N, Masuda N et al (2014) Treatment outcomes and prognostic factor for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 147:103–112
Eichler AF, Kuter I, Ryan R et al (2008) Survival in patients with brain metastases from breast cancer. Cancer 112:2359–2367
Braccini AL, Azria D, Thezenas S et al (2013) Prognostic factors of brain metastases from breast cancer: impact on targeted therapies. The Breast 22:993–998
Melisko ME, Moore DH, Sneed PK et al (2008) Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neruooncol 88:359–365
Yust-Katz S, Garciarena P, Liu D et al (2013) Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol 114:229–235
Gauthier H, Guilhaume MN, Bidard FC et al (2010) Survival on breast cancer patients with meningeal carcinomatosis. Ann Oncol 21:2183–2187
Torrejon D, Oliveira M, Cortes J et al (2013) Implication of breast cancer phenotype for patients with leptomeningeal carcinomatosis. The Breast 22:19–23
Niwinska AM, Rudnicka H, Murawska M (2011) Breast cancer carcinomatous meningitis: difference in survival depending on biological subtype, performance status, and treatment methods. J Clin Oncol 29:1073
Hayashi N, Niikura N, Masuda N et al (2015) Prognostic factor or HER2-positive breast cancer patients who develop brain metastasis: a multicenter retrospective analysis. Breast Cancer Res Treat 149:277–284
Niwinska A, Murawska M, Pogoda K (2010) Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 21:942–948
Rusnak DW, Affleck K, Cockerill SG et al (2001) The characterization of Novel, Dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res 61:7196–7203
Cameron D, Casey M, Press M et al (2008) A phase3 randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 112:533–543
Bachelot T, Romieu G, Campone M et al (2013) Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 14:64–71
Swain SM, Baselga J, Miles D et al (2014) Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase3 study CLEOPATRA. Ann Oncol 25:1116–1121
Krop IE, Lin NU, Blackwell K et al (2015) Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 26:113–119
Bartsch R, Berghoff AS, Preusser M (2014) Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J Neurooncol 116:205–206
Tsao MN, Rades D, Wirth A et al (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225
Lin NU, Winer EP (2007) Brain metastases: the HER2 paradigm. Clin Cancer Res 13:1648–1655
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomized controlled trial. Lancet Oncol 10:1037–1044
Kocher M, Soffietti R, Abacioglu U et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29:134–141
Soffietti R, Kocher M, Abacioglu UM et al (2013) A European organization for research and treatment of cancer phaseIII trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumor after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 31:65–72
Aoyama H, Tago M, Kato N et al (2007) Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 68:1388–1395
Matsunaga S, Shuto T, Kawahara N et al (2010) Gamma knife surgery for metastatic brain tumors from primary breast cancer: treatment indication based on number of tumors and breast cancer phenotype. J Neurosurg 113:65–72
Kondziolka D, Kano H, Harrison GL et al (2011) Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. J Neurosurg 114:792–800
Alexander E 3rd, Moriarty TM, Davis RB et al (1995) Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Nati Cancer Inst 87:34–40
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395
Acknowledgement
Part of this study was presented as a poster at the 2014 San Antonio Breast Cancer Symposium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Matsuo, S., Watanabe, J., Mitsuya, K. et al. Brain metastasis in patients with metastatic breast cancer in the real world: a single-institution, retrospective review of 12-year follow-up. Breast Cancer Res Treat 162, 169–179 (2017). https://doi.org/10.1007/s10549-017-4107-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4107-x