Abstract
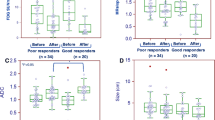
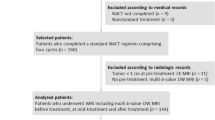
Quantitative DCE-MRI parameters including Ktrans (transfer constant min−1) can predict both response and outcome in breast cancer patients treated with neoadjuvant chemotherapy (NAC). Quantitative methods are time-consuming to calculate, requiring expensive software and interpretive expertise. For diagnostic purposes, signal intensity–time curves (SITCs) are used for tissue characterisation. In this study, we compare the ability of NAC-related changes in SITCs with Ktrans to predict response and outcomes. 73 women with primary breast cancer underwent DCE-MRI studies before and after two cycles of NAC. Patients received anthracycline and/or docetaxel-based chemotherapy. At completion of NAC, patients had local treatment with surgery & radiotherapy and further systemic treatments. SITCs for paired DCE-MRI studies were visually scored using a five-curve type classification schema encompassing wash-in and wash-out phases and correlated with Ktrans values and to the endpoints of pathological response, OS and DFS. 58 paired patients studies were evaluable. The median size by MRI measurement for 52 tumours was 38 mm (range 17–86 mm) at baseline and 26 mm (range 10–85 mm) after two cycles of NAC. Median baseline Ktrans (min−1) was 0.214 (range 0.085–0.469), and post-two cycles of NAC was 0.128 (range 0.013–0.603). SITC shapes were significantly related to Ktrans values both before (χ 2 = 43.3, P = 0.000) and after two cycles of NAC (χ 2 = 60.5, P = 0.000). Changes in curve shapes were significantly related to changes in Ktrans (χ 2 = 53.5, P = 0.000). Changes in curve shape were significantly correlated with clinical (P = 0.005) and pathological response (P = 0.005). Reductions in curve shape of ≥1 point were significant for overall improved survival using Kaplan–Meier analysis with a 5-year OS of 80.9 versus 68.6 % (P = 0.048). SITCs require no special software to generate and provide a useful method of assessing the effectiveness of NAC for primary breast cancer.





Similar content being viewed by others
References
Makhoul I, Kiwan E (2011) Neoadjuvant systemic treatment of breast cancer. J Surg Oncol 103:348–357. doi:10.1002/jso.21696
Marinovich ML, Sardanelli F, Ciatto S et al (2012) Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 21:669–677. doi:10.1016/j.breast.2012.07.006
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
Therasse P, Eisenhauer EA, Verweij J (2006) RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 42:1031–1039. doi:10.1016/j.ejca.2006.01.026
Partridge SC, Gibbs JE, Lu Y et al (2005) MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 184:1774–1781. doi:10.2214/ajr.184.6.01841774
Ah-See MLW, Makris A, Taylor NJ et al (2008) Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res 14:6580–6589. doi:10.1158/1078-0432.CCR-07-4310
Groheux D, Espie M, Giacchetti S, Hindie E (2013) Performance of FDG PET/CT in the clinical management of breast cancer. Radiology 266:388–405. doi:10.1148/radiol.12110853
Padhani AR (2011) Diffusion magnetic resonance imaging in cancer patient management. Semin Radiat Oncol 21:119–140. doi:10.1016/j.semradonc.2010.10.004
Malayeri AA, El Khouli RH, Zaheer A et al (2011) Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 31:1773–1791. doi:10.1148/rg.316115515
Sharma U, Danishad KKA, Seenu V, Jagannathan NR (2009) Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed 22:104–113. doi:10.1002/nbm.1245
Jacobs MA, Stearns V, Wolff AC et al (2010) Multiparametric magnetic resonance imaging, spectroscopy and multinuclear (23Na) imaging monitoring of preoperative chemotherapy for locally advanced breast cancer. Acad Radiol 17:1477–1485. doi:10.1016/j.acra.2010.07.009
Tozaki M, Sakamoto M, Oyama Y et al (2010) Predicting pathological response to neoadjuvant chemotherapy in breast cancer with quantitative 1H MR spectroscopy using the external standard method. J Magn Reson Imaging 31:895–902. doi:10.1002/jmri.22118
Li SP, Taylor NJ, Makris A et al (2010) Primary human breast adenocarcinoma: imaging and histologic correlates of intrinsic susceptibility-weighted MR imaging before and during chemotherapy. Radiology 257:643–652. doi:10.1148/radiol.10100421/-/DC1
Li SP, Makris A, Beresford MJ et al (2011) Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology 260:68–78. doi:10.1148/radiol.11102493
Kuhl CK, Mielcareck P, Klaschik S et al (1999) Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 211:101–110
Hayes C, Padhani AR, Leach MO (2002) Assessing changes in tumour vascular function using dynamic contrast-enhanced magnetic resonance imaging. NMR Biomed 15:154–163. doi:10.1002/nbm.756
Parker GJ, Suckling J, Tanner SF et al (1997) Probing tumor microvascularity by measurement, analysis and display of contrast agent uptake kinetics. J Magn Reson Imaging 7:564–574
d’Arcy JA, Collins DJ, Padhani AR et al (2006) Informatics in Radiology (infoRAD): Magnetic Resonance Imaging Workbench: analysis and visualization of dynamic contrast-enhanced MR imaging data. Radiographics 26:621–632. doi:10.1148/rg.262045187
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7:91–101
Fritz-Hansen T, Rostrup E, Larsson HB et al (1996) Measurement of the arterial concentration of Gd-DTPA using MRI: a step toward quantitative perfusion imaging. Magn Reson Med 36:225–231
Walker-Samuel S, Leach MO, Collins DJ (2006) Evaluation of response to treatment using DCE-MRI: the relationship between initial area under the gadolinium curve (IAUGC) and quantitative pharmacokinetic analysis. Phys Med Biol 51:3593–3602. doi:10.1088/0031-9155/51/14/021
Daniel BL, Yen YF, Glover GH et al (1998) Breast disease: dynamic spiral MR imaging. Radiology 209:499–509
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12:320–327. doi:10.1016/S0960-9776(03)00106-1
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
Orel SG (1999) Differentiating benign from malignant enhancing lesions identified at MR imaging of the breast: are time-signal intensity curves an accurate predictor? Radiology 211:5–7
Renz DM, Diekmann F, Schmitzberger FF et al (2013) Pharmacokinetic approach for dynamic breast MRI to indicate signal intensity time curves of benign and malignant lesions by using the tumor flow residence time. Invest Radiol 48:69–78. doi:10.1097/RLI.0b013e31827d29cf
El Khouli RH, Macura KJ, Kamel IR et al (2011) 3-T dynamic contrast-enhanced MRI of the breast: pharmacokinetic parameters versus conventional kinetic curve analysis. Am J Roentgenol 197:1498–1505. doi:10.2214/AJR.10.4665
Hauth EAM, Jaeger H, Maderwald S et al (2006) Evaluation of quantitative parametric analysis for characterization of breast lesions in contrast-enhanced MR mammography. Eur Radiol 16:2834–2841. doi:10.1007/s00330-006-0348-5
Kong X, Moran MS, Zhang N et al (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47:2084–2090. doi:10.1016/j.ejca.2011.06.014
Johansen R, Jensen LR, Rydland J et al (2009) Predicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRI. J Magn Reson Imaging 29:1300–1307. doi:10.1002/jmri.21778
Orton MR, d’Arcy JA, Walker-Samuel S et al (2008) Computationally efficient vascular input function models for quantitative kinetic modelling using DCE-MRI. Phys Med Biol 53:1225–1239. doi:10.1088/0031-9155/53/5/005
Acknowledgments
We acknowledge the Breast Cancer Campaign and the Breast Cancer Research Trust for providing funding for this study.
Conflict of interest
None to declare
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woolf, D.K., Padhani, A.R., Taylor, N.J. et al. Assessing response in breast cancer with dynamic contrast-enhanced magnetic resonance imaging: Are signal intensity–time curves adequate?. Breast Cancer Res Treat 147, 335–343 (2014). https://doi.org/10.1007/s10549-014-3072-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3072-x