Abstract
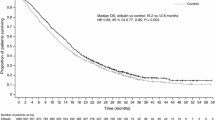
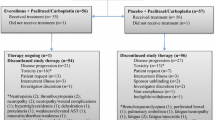
This multicenter, open-label, randomized phase II trial compared the efficacy and tolerability of weekly ixabepilone versus the standard 3 weekly dosing regimen. Patients with human epidermal growth factor receptor 2-negative, metastatic breast cancer (MBC) were randomly assigned to receive either ixabepilone 16 mg/m2 as a 1-h intravenous (IV) infusion weekly on days 1, 8, and 15 of a 28-day cycle (1 week off therapy; n = 85), or 40 mg/m2 as a 3-h IV infusion on day 1 of a 21-day cycle (n = 91), until disease progression or unacceptable toxicity. Randomization was stratified by (i) measurable versus nonmeasurable (evaluable) disease, (ii) ≤two versus >two prior chemotherapy regimens for MBC, and (iii) hormone receptor (HR)-positive versus HR-negative breast cancer. The primary endpoint was rate of progression-free survival (PFS) at 6 months. Of 176 randomized patients, 171 were treated. The 6-month PFS rate was significantly higher in patients treated with ixabepilone every 3 weeks (42.7, 95 % confidence interval [CI] 31.5–53.5) compared with those who received ixabepilone weekly (28.6, 95 % CI 18.9–38.9; log-rank P = 0.03). Every-3-week dosing significantly prolonged median PFS versus weekly dosing (5.3 vs. 2.9 months; log-rank P = 0.05). The every-3-week regimen was associated with higher rates of grade 3/4 toxicities, particularly neutropenia (38.2 vs. 6.1 %) and a higher rate of patient withdrawal due to adverse events. These results suggest that every-3-week ixabepilone is more effective than weekly treatment in MBC, albeit with more toxicity.



Similar content being viewed by others
References
Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM (1995) Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 55:2325–2333
Fumoleau P, Coudert B, Isambert N, Ferrant E (2007) Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol 18(Suppl 5):v9–v15
Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC, Kramer RA (2001) BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 7:1429–1437
Ayoub JP, Verma Sh, Verma S (2012) Advances in the management of metastatic breast cancer: options beyond first-line chemotherapy. Curr Oncol 19:91–105. doi:10.3747/co.19.1024
Lee FY, Smylka R, Johnston K, Menard K, McGlinchey K, Peterson RW, Wiekesiek A, Vite G, Fairchild CR, Kramer R (2009) Preclinical efficacy spectrum and pharmacokinetics of ixabepilone. Cancer Chemother Pharmacol 63:201–212. doi:10.1007/s00280-008-0727-5
Rivera E, Lee J, Davies A (2008) Clinical development of ixabepilone and other epothilones in patients with advanced solid tumors. Oncologist 13:1207–1223. doi:10.1634/theoncologist.2008-0143
Ferrandina G, Mariani M, Andreoli M, Shahabi S, Scambia G, Ferlini C (2012) Novel drugs targeting microtubules: the role of epothilones. Curr Pharm Des 18:2793–2803
Thomas ES, Gomez HL, Li RK, Chung HC, Fein LE, Chan VF, Jassem J, Pivot XB, Klimovsky JV, de Mendoza Gh, Xu B, Campone M, Lerzo GL, Peck RA, Mukhopadhyay P, Vahdat LT, Roché HH (2007) Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25:5210–5217
Hortobagyi GN, Gomez HL, Li RK, Chung HC, Fein LE, Chan VF, Jassem J, Lerzo GL, Pivot XB, Hurtado de Mendoza F, XuB, Vahdat LT, Peck RA, Mukhopadhyay P, Roché HH (2010) Analysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanes. Breast Cancer Res Treat 122:409-418. doi: 10.1007/s10549-010-0901-4
Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, Da Costa SC, Ro J, Rubio G, Rondinon M, Perez Manga G, Peck R, Poulart V, Conte P (2010) Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 28:3256–3263. doi:10.1200/JCO.2009.24.4244
National Comprehensive Cancer Network. NCCN Clinical Guidelines in Oncology (NCCN Guidelines®) Version 1.2013: Breast Cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Perez EA, Lerzo G, Pivot X, Thomas E, Vahdat L, Bosserman L, Viens P, Cai C, Mullaney B, Peck R, Hortobagyi GN (2007) Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol 25:3407–3414
Thomas E, Tabernero J, Fornier M, Conté P, Fumoleau P, Lluch A, Vahdat LT, Bunnell CA, Burris HA, Viens P, Baselga J, Rivera E, Guarneri V, Poulart V, Klimovsky J, Lebwohl D, Martin M (2007) Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol 25:3399–3406
Cristofanilli M (2012) Advancements in the treatment of metastatic breast cancer (MBC): the role of ixabepilone. J Oncol 703858. Published online May 8 2012. doi:10.1155/2012/703858
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649. doi:10.1200/JCO.2007.11.6699
Moulder S, Li H, Wang M, Gradishar WJ, Perez EA, Sparano JA, Pins M, Yang X, Sledge GW (2010) A phase II trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with HER2-positive metastatic breast cancer: an Eastern Cooperative Oncology Group Trial. Breast Cancer Res Treat 119:663–671. doi:10.1007/s10549-009-0658-9
Rugo HS, Campone M, Amadori D, Wardley AM, Aldrighetti D, Conte PF, Liu D, Mudenda B, McHenry MB, Pivot XB (2010) Randomized phase II study of weekly versus every 3 week ixabepilone plus bevacizumab (ixa/bev) versus paclitaxel plus bev (pac/bev) as first-line therapy for metastatic breast cancer (MBC): final results. J Clin Oncol 28(Suppl;abstr1040):15s
Kossoff EB, Ngamphaiboon N, Laudico TJ, O’Connor TL (2011) Weekly ixabepilone administration in heavily pretreated metastatic breast cancer patients. Med Oncol 28(Suppl 1):S115–S120. doi:10.1007/s12032-010-9726-6
National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) v3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
Schröder CP, de Munck L, Westermann AM, Smit WM, Creemers GJ, de Graaf H, Stouthard JM, van Deijk G, Erjavec Z, van Bochove A, Vader W, Willemse PH (2011) Weekly docetaxel in metastatic breast cancer patients: no superior benefits compared to three-weekly docetaxel. Eur J Cancer 47:1355–1362. doi:10.1016/j.ejca.2010.12.018
Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Mayer EL, Naughton M, Layman RM, Carey LA, Somer RA, Perez EA, Hudis C, Winer EP (2012) CALGB 40502/NCCTG N063H: randomized phase III trial of weekly paclitaxel (P) compared to weekly nanoparticle albumin bound nab-paclitaxel (NP) or ixabepilone (Ix) with or without bevacizumab (B) as first-line therapy for locally recurrent or metastatic breast cancer (MBC). J Clin Oncol 30(Suppl;abstr CRA1002):18s
Awada A, Piccart MJ, Jones SF, Peck RA, Gil T, Lebwohl D, Wu CY, Burris HA III (2009) Phase I dose escalation study of weekly ixabepilone, an epothilone analog, in patients with advanced solid tumors who have failed standard therapy. Cancer Chemother Pharmacol 63:417–425. doi:10.1007/s00280-008-0751-5
Acknowledgments
The authors thank all physicians at the participating US Oncology network sites where this study was conducted. They also thank all nurses, in particular Michelle H. Ellenwood, Marjorie Bennett, Valerie Francis, and Kirsten Stuber, who contributed to the success of this clinical trial. The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoint and scientific expertise. They wish to acknowledge StemScientific, funded by Bristol-Myers Squibb, for providing writing and editorial support.
Conflict of interest
John W. Smith II, Svetislava Vukelja, Amy Rabe, Nicole Wentworth-Hartung, Nicholas Koutrelakos, Spencer H Shao, Thomas Whittaker, Yunfei Wang, and Lina Asmar declare that they have no conflict of interest. Joyce O’Shaughnessy has received remuneration from Bristol-Myers Squibb (Speakers Bureau). Diane Opatt McDowell and Pralay Mukhopadhyay are employees and stock owners of Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Additional information
Previous presentations: J. W. Smith, S. J. Vukelja, A. C. Rabe, et al. Final results of a phase II randomized trial of weekly or every-3-week ixabepilone in metastatic breast cancer (MBC). The American Society of Clinical Oncology Breast Cancer Symposium, 2010 (abstr 268).
Rights and permissions
About this article
Cite this article
Smith, J.W., Vukelja, S., Rabe, A. et al. Phase II randomized trial of weekly and every-3-week ixabepilone in metastatic breast cancer patients. Breast Cancer Res Treat 142, 381–388 (2013). https://doi.org/10.1007/s10549-013-2742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2742-4