Abstract
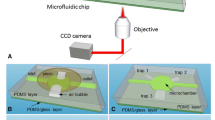
In this study, fine bubbles were successfully generated and used as a simple, low-cost driving force for mixing fluids in an integrated microfluidic bead-based enzyme-linked immunosorbent assay (ELISA) to rapidly and quantitatively detect apolipoprotein A1 (APOA1), a biomarker highly correlated with bladder cancer. A wooden gas diffuser was embedded underneath a microfluidic chip to refine injected air and generate bubbles of less than 0.3 mm. The rising bubbles caused disturbances and convection in the fluid, increasing the probability of analyte interaction. This setup not only simplifies the micromixer design but also achieves rapid mixing with a small airflow as a force. We used this bubble-driven micromixer in a bead-based ELISA that targeted APOA1. The results indicate that this micromixer reduced the time for each incubation from 60 min in the conventional assay to 8 min with the chip, resulting in a reduction of total ELISA reaction time from 3–4 h to 30–40 min. Furthermore, the concentration detection limit was 9.16 ng/mL, which was lower than the detection cut-off value (11.16 ng/mL) for bladder cancer diagnosis reported in the literature. Therefore, this chip can be used to achieve rapid low-cost bladder cancer detection and may be used in point-of-care cancer monitoring.







Similar content being viewed by others
References
D. Ahmed, X. Mao, B.K. Juluri, T.J. Huang, Microfluid. Nanofluid. 7, 727 (2009)
P.A. Auroux, D. Iossifidis, D.R. Reyes, A. Manz, Anal. Chem. 74, 2637 (2002)
Y.T. Chen, C.L. Chen, H.W. Chen, T. Chung, C.C. Wu, C.D. Chen, C.W. Hsu, M.C. Chen, K.H. Tsui, P.L. Chang, Y.S. Chang, J.S. Yu, J. Proteome Res. 9, 5803 (2010)
Y.T. Chen, H.W. Chen, D. Domanski, D.S. Smith, K.H. Liang, C.C. Wu, C.L. Chen, T. Chung, M.C. Chen, Y.S. Chang, C.E. Parker, C.H. Borchers, J.S. Yu, J. Proteomics 75, 3529 (2012)
P. Garstecki, M.J. Fuerstman, M.A. Fischbach, S.K. Sia, G.M. Whitesides, Lab Chip 6, 207 (2006)
Y. Hayashi, R. Matsuda, T. Maitani, Anal. Chem. 76, 1295 (2004)
M. Herrmann, E. Roy, T. Veres, M. Tabrizian, Lab Chip 7, 1546 (2007)
P.I. Karakiewicz, S. Benayoun, C. Zippe, G. Lüdecke, H. Boman, M. Sanchez-Carbayo, R. Casella, C. Mian, M.G. Friedrich, S. Eissa, H. Akaza, H. Huland, H. Hedelin, R. Rupesh, N. Miyanaga, A.I. Sagalowsky, M.J. Marberger, S.F. Shariat, BJU Int. 97, 997 (2006)
D.S. Kim, S.H. Lee, T.H. Kwon, C.H. Ahn, Lab Chip 5, 739 (2005)
D.S. Kim, S.W. Lee, T.H. Kwon, S.S. Lee, J. Micromech. Microeng. 14, 798 (2004)
H. Li, C. Li, H. Wu, T. Zhang, J. Wang, S. Wang, J. Chang, Proteome Sci. 9, 1 (2011)
Y.H. Lin, Y.J. Chen, C.S. Lai, Y.T. Chen, C.L. Chen, J.S. Yu, Y.S. Chang, Biomicrofluidics 7, 024103 (2013)
M. Lindén, S.B. Lind, C. Mayrhofer, U. Segersten, K. Wester, Y. Lyutvinskiy, R. Zubarev, P.-U. Malmström, U. Pettersson, Proteomics 12, 135 (2012)
R.H. Liu, J. Yang, M.Z. Pindera, M. Athavale, P. Grodzinski, Lab Chip 2, 151 (2002)
R.H. Liu, R. Lenigk, R.L. Druyor-Sanchez, J. Yang, P. Grodzinski, Anal. Chem. 75, 1911 (2003)
Y. Lotana, C.G. Roehrborna, Urology 61, 109 (2003)
L.H. Lu, K.S. Ryu, C. Liu, J. Microelectromech. Syst. 11, 462 (2002)
X. Mao, B.K. Juluri, M.I. Lapsley, Z.S. Stratton, T.J. Huang, Microfluid. Nanofluid. 8, 139 (2010)
K.G. McKenzie, L.K. Lafleur, B.R. Lutz, P. Yager, Lab Chip 9, 3543 (2009)
D. Mitropoulos, A. Kiroudi-Voulgari, P. Nikolopoulos, T. Manousakas, A. Zervas, J. Endourol. 19, 861 (2005)
L.V. Rajakovic, D.D. Markovic, V.N. Rajakovic-Ognjanovic, D.Z. Antanasijevic, Talanta 102, 79 (2012)
H. Suzuki, C.M. Ho, N. Kasagi, J. Microelectromech. Syst. 13, 779 (2004)
T. Tanahashi, Y. Matsumura, H. Ohmori, T. Tanaka, Acta Med. Okayama 32, 139 (1978)
J.H. Tsai, L. Lin, J. Microelectromech. Syst. 11, 665 (2002)
R.A. Vijayendran, K.M. Motsegood, D.J. Beebe, D.E. Leckband, Langmuir 19, 1824 (2003)
H. Xiao, D. Liang, G. Liu, M. Guo, W. Xing, J. Cheng, Lab Chip 6, 1067 (2006)
S.Y. Yang, J.L. Lin, G.B. Lee, J. Micromech. Microeng. 19, 035020 (2009a)
Y.N. Yang, H.I. Lin, J.H. Wang, S.C. Shiesh, G.B. Lee, Biosens. Bioelectron. 24, 3091 (2009b)
Acknowledgments
The authors would like to thank the National Science Council and the Ministry of Education of Taiwan for the financial support provided to this study under Grant No. NSC 100-2221-E-182-021-MY3 and EMRPD1A0761.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, YH., Wang, CC. & Lei, K.F. Bubble-driven mixer integrated with a microfluidic bead-based ELISA for rapid bladder cancer biomarker detection. Biomed Microdevices 16, 199–207 (2014). https://doi.org/10.1007/s10544-013-9822-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-013-9822-4