Abstract
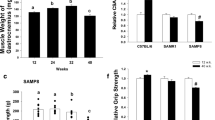
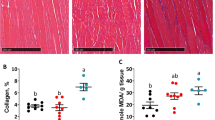
Sarcopenia is a condition of the loss of muscle mass that is associated with aging and that increases the risk for bedridden state, thereby warranting studies of interventions that attenuate sarcopenia. Here the effects of 2-month dietary l-lysine (Lys) supplementation (1.5–3.0 %) on myofibrillar protein degradation and major proteolytic systems were investigated in senescence-accelerated mouse prone 8 (SAMP8). At 36 weeks of age, skeletal muscle and lean body mass was reduced in SAMP8 when compared with control senescence-accelerated mouse resistant 1 (SAMR1). The myofibrillar protein degradation, which was evaluated by the release of 3-methylhistidine, was stimulated in SAMP8, and the autophagy activity, which was evaluated by light chain 3-II, was stimulated in the skeletal muscle of SAMP8. The activation of ubiquitin-proteasome system was not observed in the muscles of SAMP8. However, myofibrillar protein degradation and autophagic activity in skeletal muscles of SAMP8 were suppressed by dietary intake of 3.0 % Lys. The present data indicate that myofibrillar protein degradation by bulk autophagy is stimulated in the skeletal muscles of SAMP8 and that dietary Lys supplementation attenuates sarcopenia in SAMP8 by suppressing autophagic proteolysis.



Similar content being viewed by others
Abbreviations
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- LC3:
-
Light chain 3
- Lys:
-
l-Lysine
- MeHis:
-
3-Methylhistidine
- mTOR:
-
Mammalian target of rapamycin
- Mul1:
-
Mitochondrial E3 ubiquitin protein ligase 1
- MuRF1:
-
Muscle ring-finger protein 1
- PGC-1α:
-
Peroxisome proliferator activated receptor γ co-activator 1α
- qRT-PCR:
-
Quantitative reverse transcription PCR
- SAMP8:
-
Senescence-accelerated mouse prone 8
- SAMR1:
-
Senescence-accelerated mouse resistant 1
- S6K1:
-
p70 ribosomal protein S6 kinase 1
- UPS:
-
Ubiquitin-proteasome system
- 4E-BP1:
-
Eukaryotic initiation factor 4E binding protein 1
References
Altun M, Besche HC, Overkleeft HS, Piccirillo R, Edelmann MJ, Kessler BM, Goldberg AL, Ulfhake B (2010) Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem 285:39597–395608
Angcajas AB, Hirai N, Kaneshiro K, Karim MR, Horii Y, Kubota M, Fujimura S, Kadowaki M (2014) Diversity of amino acid signaling pathways on autophagy regulation: a novel pathway for arginine. Biochem Biophys Res Commun 446:8–14
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of post absorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419
Børsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR (2008) Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr 27:189–195
Chalil S, Pierre N, Bakker AD, Manders RJ, Pletsers A, Francaux M, Klein-Nulend J, Jaspers RT, Deldicque L (2015) Aging related ER stress is not responsible for anabolic resistance in mouse skeletal muscle. Biochem Biophys Res Commun 468:702–707
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424
Derave W, Eijnde BO, Ramaekers M, Hespel P (2005) Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp Gerontol 40:562–572
Dong W, Quo W, Wang F, Li C, Xie Y, Zheng X, Shi H (2015) Electroacupuncture upregulates SIRT1-dependent PGC-1α expression in SAMP8 mice. Med Sci Monit 21:3356–3362
Edström E, Altun M, Hägglund M, Ulfhake B (2006) Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol Ser A 61:663–674
Fan J, Kou X, Jia S, Yang X, Yang Y, Chen N (2016) Autophagy as a potential target for sarcopenia. J Cell Physiol 231:1450–1459
Foletta VC, White LJ, Larsen AE, Léger B, Russell AP (2011) The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch 461:325–335
García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P (2016) Autophagy maintains stemness by preventing senescence. Nature 529:37–42
Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP, Garduno-Garcia Jde J, Abdul-Ghani M, Richardson A, DeFronzo RA, Mandarino L, Van Remmen H, Musi N (2011) Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 60:2051–2060
Guo AY, Leung KS, Siu PM, Qin JH, Chow SK, Qin L, Li CY, Cheung WH (2015) Muscle mass, structural and functional investigations of senescence-accelerated mouse P8 (SAMP8). Exp Anim 64:425–433
Hatazawa Y, Senoo N, Tadaishi M, Ogawa Y, Ezaki O, Kamei Y, Miura S (2015) Metabolomic analysis of the skeletal muscle of mice overexpressing PGC-1α. PLoS ONE 10:e0129084
Hughes DC, Stewart CE, Sculthorpe N, Dugdale HF, Yousefian F, Lewis MP, Sharples AP (2016) Testosterone enables growth and hypertrophy in fusion impaired myoblasts that display myotube atrophy: deciphering the role of androgen and IGF-I receptors. Biogerontology 17:619–639
Itakura E, Kishi C, Inoue K, Mizushima N (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19:5360–5372
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296
Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C (2012) The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 11:801–809
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Kirkin V, McEwan DG, Novak I, Dikic I (2009) A role for ubiquitin in selective autophagy. Mol Cell 34:259–269
Komatsu M, Ichimura Y (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 584:1374–1378
Lamont LS, McCullough AJ, Kalhan SC (2001) Gender differences in leucine, but not lysine, kinetics. J Appl Physiol 91:357–362
Liu HW, Chan YC, Wang MF, Wei CC, Chang SJ (2015) Dietary (-)-epigallocatechin-3-gallate supplementation counteracts aging-associated skeletal muscle insulin resistance and fatty liver in senescence-accelerated mouse. J Agric Food Chem 63:8407–8417
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Nagasawa T, Hirano J, Yoshizawa F, Nishizawa N (1998) Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Biosci Biotechnol Biochem 62:1932–1937
Narici MV, Maffulli N (2010) Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95:139–159
Pagano TB, Wojcik S, Costagliola A, De Biase D, Iovino S, Iovane V, Russo V, Papparella S, Paciello O (2015) Age related skeletal muscle atrophy and upregulation of autophagy in dogs. Vet J 206:54–60
Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M (2012) Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr 31:583–601
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Russ DW, Boyd IM, McCoy KM, McCorkle KW (2016) Muscle-specificity of age-related changes in markers of autophagy and sphingolipid metabolism. Biogerontology 16:747–759
Sakuma K, Kinoshita M, Ito Y, Aizawa M, Aoi W, Yamaguchi A (2016) p62/SQSTM1 but not LC3 is accumulated in sarcopenic muscle of mice. J Cachexia Sarcopenia Muscle 7:204–212
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Muñoz-Cánoves P, Musarò A, Pende M, Reggiani C, Rizzuto R, Schiaffino S (2013) Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14:303–323
Sato T, Ito Y, Nagasawa T (2013) Regulation of skeletal muscle protein degradation and synthesis by oral administration of lysine in rats. J Nutr Sci Vitaminol 59:412–419
Sato T, Ito Y, Nagasawa T (2014) Lysine suppresses myofibrillar protein degradation by regulating the autophagic-lysosomal system through phosphorylation of Akt in C2C12 cells. Springerplus 3:584
Sato T, Ito Y, Nagasawa T (2015) Dietary l-lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low-protein diet. J Agric Food Chem 63:8192–8198
Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, Rudd JA, Siu PM (2015) Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signalling in aged skeletal muscle. Acta Diabetol 52:1063–1075
Sugawara T, Ito Y, Nishizawa N, Nagasawa T (2009) Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 37:609–616
Takeda T (1999) Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging 20:105–110
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J 19:94–102
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT (2009) Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA 106:20405–20410
White Z, White RB, McMahon C, Grounds MD, Shavlakadze T (2016) High mTORC1 signaling is maintained, while protein degradation pathways are perturbed in old murine skeletal muscles in the fasted state. Int J Biochem Cell Biol 78:10–21
Young VR, Munro HN (1978) Nτ–methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc 37:2291–3000
Yun J, Puri R, Yang H, Lizzio MA, Wu C, Sheng ZH, Guo M (2014) MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. Elife 3:e01958
Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6:472–483
Acknowledgments
Amino acids were provided by Ajinomoto Co., Inc. Primer sequences for atrogin-1 and MuRF1 were provided by Professor Takeshi Nikawa, Tokushima University, Japan.
Funding
This work was supported by the Sasakawa Scientific Research Grant from The Japan Science Society (Grant No. 27-414).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest related to this study.
Informed consent
Human subject is not involved in this study.
Research involving Animals
Male SAMP8 (15-week-old, 27–35 g, n = 17) and SAMR1 (15-week-old, 31–35 g, n = 6) were purchased from Japan SLC, Inc. (Shizuoka, Japan). All animal protocols were approved by the Iwate University Animal Research Committee and were performed in compliance with the Guidelines for Animal Experiments of Iwate University (approval number, A201517).
Rights and permissions
About this article
Cite this article
Sato, T., Ito, Y. & Nagasawa, T. l-Lysine suppresses myofibrillar protein degradation and autophagy in skeletal muscles of senescence-accelerated mouse prone 8. Biogerontology 18, 85–95 (2017). https://doi.org/10.1007/s10522-016-9663-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-016-9663-7