Abstract
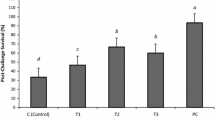
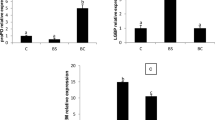
Immunoprophylactic alternatives are needed to fight against acute hepatopancreatic necrosis disease (AHPND). This study addressed the effects of different routes of Bacillus administration (via diet or culture water) on growth performance, survival, and immune parameters of Litopenaeus vannamei challenged with Vibrio parahaemolyticus-AHPND. Bacillus mixture and Bacillus licheniformis BCR 4-3 were added to feed (1 × 106 CFU g−1) or rearing water (1 × 106, 2 × 106, 3 × 106 CFU L−1). Animals were challenged with a determined median lethal V. parahaemolyticus concentration. Survival, growth performance, and immune response were determined. The results showed that B. licheniformis BCR 4-3 (3 × 106 CFU L−1) administration via culture water significantly (P < 0.05) increased survival (53.4%) in shrimp challenged with V. parahaemolyticus; growth performance was also significantly (P < 0.05) higher compared to the control group. Moreover, the relative expression of innate immune-related genes, such as lysozyme, penaeidin4, crustin, and superoxide dismutase, significantly (P < 0.05) upregulated in shrimp fed B. licheniformis BCR4-3 compared with the control group. In conclusion, the administration of probiotic B. licheniformis BCR4-3 in feed and culture water increased resistance in L. vannamei against V. parahaemolyticus-AHPND and was found associated with an upregulation of key antimicrobial innate immune-related genes.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aderaldo-Vidal JM, da Cruz MN, dos Santos FL, Mendes PP, Mendes ES (2018) Probiotic potential of Bacillus cereus against Vibrio spp. in post-larvae shrimps. Revista Caatinga 18:495–503. https://doi.org/10.1590/1983-21252018v31n226rc
Amend DF (1981) Potency testing of fish vaccines. Fish biologics: serodiagnostics and vaccines. Developments in Biological Standardization 49:447–454
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research 64:5245–5250. https://doi.org/10.1158/0008-5472.
Antony SP, Singh IB, Sudheer NS, Vrinda S, Priyaja P, Philip R (2011) Molecular characterization of a crustin-like antimicrobial peptide in the giant tiger shrimp, Penaeus monodon, and its expression profile in response to various immunostimulants and challenge with WSSV. Immunobiology 216:184–194. https://doi.org/10.1016/j.imbio.2010.05.030
Bej AK, Patterson DP, Brashera CW, Vickerya MCL, Jones DD, Kaysner CA (1999) Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. Journal of Microbiological Methods. 36:215–225. https://doi.org/10.1016/S0167-7012(99)00037-8
Burge EJ, Madigan DJ, Burnett LE, Burnett KG (2007) Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish & Shellfish Immunology 22:327–339. https://doi.org/10.1016/j.fsi.2006.06.004
Campa-Córdova AI, Hernández-Saavedra NY, Ascencio F (2002) Superoxide dismutase as modulator of immune function in American white shrimp (Litopenaeus vannamei). Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology 133:557–565. https://doi.org/10.1016/S1532-0456(02)00125-4
Cuthbertson BJ, Yang Y, Bachère E, Büllesbach EE, Gross PS, Aumelas A (2005) Solution structure of synthetic penaeidin-4 with structural and functional comparisons with penaeidin-3. Journal of Biological Chemistry 280:16,009–16,018. https://doi.org/10.1074/jbc.M412420200
De Lorgeril J, Gueguen Y, Goarant C, Goyard E, Mugnier C, Fievet J, Piquemal D, Bachere E (2008) A relationship between antimicrobial peptide gene expression and capacity of a selected shrimp line to survive a Vibrio infection. Molecular Immunology 45:3438–3445. https://doi.org/10.1016/j.molimm.2008.04.002
Destoumieux, D., Bulet, P., Loew, D., Van Dorsselaer, A., Rodriguez, J., & Bachere, E. (1997). Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). Journal of Biological Chemistry, 272, 28,398–28,406. DOI https://doi.org/10.1074/jbc.272.45.28398
Elmahdi S, DaSilva LV, Parveen S (2016) Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiology 57:128–134. https://doi.org/10.1016/j.fm.2016.02.008
Escamilla-Montes R, Luna-González A, Flores-Miranda MC, Álvarez-Ruiz P, Fierro-Coronado JA, Sánchez-Ortiz AC, Ávila-Leal J (2015) Isolation and characterization of potential probiotic bacteria suitable for mollusk larvae cultures. Thai Journal of Veterinary Medicine 45:11–21
FAO. (2019) FAO yearbook. Fishery and Aquaculture Statistics 2017/FAO annuaire. Statistiques des pêches et de l’aquaculture 2017/FAO anuario. Estadísticas de pesca y acuicultura 2017. Rome/Roma. ISBN 978–92–5-131,669-6.
Finney DJ, Tattersfield F (1952) Probit analysis. In: Cambridge University Press. USA, Cambridge, New York 318 pp
González-Romero MA, Hernandez-Llamas A, Ruiz-Velazco JM, Plascencia-Cuevas TN, Nieto-Navarro JT (2014) Stochastic bio-economic optimization of pond size for intensive commercial production of whiteleg shrimp Litopenaeus vannamei. Aquaculture 433:496–503. https://doi.org/10.1016/j.aquaculture.2014.07.010
Gupta A, Verma G, Gupta P (2016) Growth performance, feed utilization, digestive enzyme activity, innate immunity and protection against Vibrio harveyi of freshwater prawn, Macrobrachium rosenbergii fed diets supplemented with Bacillus coagulans. Aquaculture international 24(5):1379–1392. https://doi.org/10.1007/s10499-016-9996-x
Hai NV (2015) The use of probiotics in aquaculture. Journal of Applied Microbiology 119:917–935. https://doi.org/10.1111/jam.12886
Hao K, Liu JY, Ling F, Liu XL, Lu L, Xia L, Wang GX (2014) Effects of dietary administration of Shewanella haliotis D4, Bacillus cereus D7 and Aeromonas bivalvium D15, single or combined, on the growth, innate immunity and disease resistance of shrimp, Litopenaeus vannamei. Aquaculture 428:141–149. https://doi.org/10.1016/j.aquaculture.2014.03.016
Hellemans J, Mortier G, De Paepe AF, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology 8:R19. https://doi.org/10.1186/gb-2007-8-2-r19
Hoseinifar SH, Dadar M, Ringø E (2017) Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: the functional feed additives scenario. Aquaculture Research 48:3987–4000. https://doi.org/10.1111/are.13368
Hoseinifar SH, Sun Y, Wang A, Zhou Z (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Frontiers in Microbiology 9:2429. https://doi.org/10.3389/fmicb.2018.02429
Jollès P, Jollès J (1984) What’s new in lysozyme research? Always a model system, today as yesterday. Molecular & Cellular Biochemistry 63:165–189. https://doi.org/10.1007/BF00285225
Kewcharoen W, Srisapoome P (2019) Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish & Shellfish Immunology 94:175–189. https://doi.org/10.1016/j.fsi.2019.09.013
Lane DJ (1991) 16S/23S rRNA sequencing. In: Goodfellow M, Wiley J (eds) Nucleic acid techniques in bacterial systematic E Stackebrandt. USA, New York, pp 115–175
Laranja JLQ, Ama EC, Ludevese-Pascual GL, Niu Y, Geaga MJ, Schryver PD, Bossier P (2017) A probiotic Bacillus strain containing amorphous poly-beta-hydroxybutyrate (PHB) stimulates the innate immune response of Penaeus monodon postlarvae. Fish & Shellfish Immunology 68:2002–2010. https://doi.org/10.1016/j.fsi.2017.07.023
Lazado CC, Caipang CMA, Estante EG (2015) Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish & Shellfish Immunology 45:2–12. https://doi.org/10.1016/j.fsi.2015.02.023
Li CY, Song YL (2010) Proline-rich domain of penaeidin molecule exhibits autocrine feature by attracting penaeidin-positive granulocytes toward the wound-induced inflammatory site. Fish & Shellfish Immunology 29:1044–1052. https://doi.org/10.1016/j.fsi.2010.08.020
Li M, Ma C, Li H, Peng J, Zeng D, Chen X, Li C (2018) Molecular cloning, expression, promoter analysis and functional characterization of a new Crustin from Litopenaeus vannamei. Fish & Shellfish Immunology 73:42–49. https://doi.org/10.1016/j.fsi.2017.12.002
Lim SY, Loo KW, Wong WL (2019) Synergistic antimicrobial effect of a seaweed-probiotic blend against acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Probiotics & Antimicrobial Proteins:1–12. https://doi.org/10.1007/s12602-019-09616-8.
Lin YC, Chen JC, Chen YY, Yeh ST, Chen LL, Huang CL, Li CC (2015) Crowding of white shrimp Litopenaeus vananmei depresses their immunity to and resistance against Vibrio alginolyticus and white spot syndrome virus. Fish & Shellfish Immunology 45(1):104–111. https://doi.org/10.1016/j.fsi.2015.02.012
Liu KF, Chiu CH, Shiu YL, Cheng W, Liu CH (2010) Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish & Shellfish Immunology 28:837–844. https://doi.org/10.1016/j.fsi.2010.01.012
Liu H, Li Z, Tan B, Lao Y, Duan Z, Sun W, Dong X (2014) Isolation of a putative probiotic strain S12 and its effect on growth performance, non-specific immunity and disease-resistance of white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology 41:300–307. https://doi.org/10.1016/j.fsi.2014.08.028
Liu XF, Li Y, Li JR, Cai LY, Li XX, Chen JR, Lyu SX (2015) Isolation and characterisation of Bacillus spp. antagonistic to Vibrio parahaemolyticus for use as probiotics in aquaculture. World Journal of Microbiology & Biotechnology 31:795–803. https://doi.org/10.1007/s11274-015-1833-2
López-León P, Luna-González A, Escamilla-Montes R, Flores-Miranda MDC, Fierro-Coronado JA, Álvarez-Ruiz P, Diarte-Plata G (2016) Isolation and characterization of infectious Vibrio parahaemolyticus, the causative agent of AHPND, from the whiteleg shrimp (Litopenaeus vannamei). Latin American Journal of Aquatic Research 44:470–479. https://doi.org/10.3856/vol44-issue3-fulltext-5
Luna-González A, Moreno-Herrera JT, Campa-Córdova AI, González-Ocampo HA, Fierro-Coronado JA, Álvarez-Ruíz P, Bueno-Ibarra MA (2013) Immune response and gene expression of white shrimp (Litopenaeus vannamei) induced by microbial immunostimulants. Latin American Journal of Aquatic Research 41:898–890. https://doi.org/10.3856/vol41-issue5-fulltext-10
Madani NSH, Adorian TJ, Ghafari Farsani H, Hoseinifar SH (2018) The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquaculture Research 49:1926–1933. https://doi.org/10.1111/are.13648
Moriarty D (1998) Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 164:351–358. https://doi.org/10.1016/S0044-8486(98)00199-9
Nimrat S, Suksawat S, Boonthai T, Vuthiphandchai V (2012) Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Veterinary Microbiology 159:443–450. https://doi.org/10.1016/j.vetmic.2012.04.029
Olmos J, Acosta M, Mendoza G, Pitones V (2020) Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Archives of Microbiology 202(3):427–435. https://doi.org/10.1007/s00203-019-01757-2
Peraza-Gómez V, Luna-González A, Campa-Córdova AI, López-Meyer M, Fierro-Coronado JA, Álvarez-Ruiz P (2009) Probiotic microorganisms and antiviral plants reduce mortality and prevalence of WSSV in shrimp (Litopenaeus vannamei) cultured under laboratory conditions. Aquaculture Research 40:1481–1489. https://doi.org/10.1111/j.1365-2109.2009.02248.x
Pessione E (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Frontiers in Cellular & Infection Microbiology 2(86). https://doi.org/10.3389/fcimb.2012.00086
Ravi AV, Musthafa KS, Jegathammbal G, Kathiresan K, Pandian SK (2007) Screening and evaluation of probiotics as a biocontrol agent against pathogenic Vibrios in marine aquaculture. Letters in Applied Microbiology 45:219–223. https://doi.org/10.1111/j.1472-765X.2007.02180.x
Sánchez-Ortiz AC, Angulo C, Luna-González A, Álvarez-Ruiz P, Mazón-Suástegui JM, Campa-Córdova ÁI (2016) Effect of mixed-Bacillus spp. isolated from pustulose ark Anadara tuberculosa on growth, survival, viral prevalence and immune-related gene expression in shrimp Litopenaeus vannamei. Fish & Shellfish Immunology 59:95–102. https://doi.org/10.1016/j.fsi.2016.10.022
Schnapp D, Kemp GD, Smith VJ (1996) Purification and characterization of a proline-rich antibacterial peptide, with sequence similarity to bactenecin-7, from the haemocytes of the shore crab Carcinus maenas. European Journal of Biochemistry 240:532–539. https://doi.org/10.1111/j.1432-1033.1996.0532hx
Sekar A, Packyam M, Kim K (2016) Growth enhancement of shrimp and reduction of shrimp infection by Vibrio parahaemolyticus and white spot syndrome virus with dietary administration of Bacillus sp. Mk22. Microbiology & Biotechnology Letters 44:261–267. https://doi.org/10.4014/mbl.1605.05001
Shen WY, Fu LL, Li WF, Zhu YR (2010) Effect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp (Litopenaeus vannamei). Aquaculture Research 41:1691–1698. https://doi.org/10.1111/j.1365-2109.2010.02554.x
Sirikharin, R., S. Taengchaiyaphum, K. Sritunyalucksana, S. Thitamadee, T.W. Flegel & R. Mavichak. (2014). A new and improved PCR method for detection of AHPND bacteria. [http://www.enaca.org/modules/ news/article.php?article_id = 2030&title = new620].pc r-detection-method-for-ahpnd bacteria.
Soltani M, Ghosh K, Hoseinifar SH, Kumar V, Lymbery AJ, Roy S, Ringø E (2019) Genus Bacillus, promising probiotics in aquaculture: aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Reviews in Fisheries Science & Aquaculture 27:331–379. https://doi.org/10.1080/23308249.2019.1597010
Tepaamorndech S, Chantarasakha K, Kingcha Y, Chaiyapechara S, Phromson M, Sriariyanun M, Visessanguan W (2019) Effects of Bacillus aryabhattai TBRC8450 on vibriosis resistance and immune enhancement in Pacific white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology 86:4–13. https://doi.org/10.1016/j.fsi.2018.11.010
Valenti WC, Kimpara JM, Preto BL (2011) Measuring aquaculture sustainability. World Aquaculture 42:26–30
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., & Speleman, F. (2002) Accurate normalization of real-time quantitative RT-CR data by geometric averaging of multiple internal control genes. Genome Biology, 3, research0034.1–research0034.11.https://doi.org/10.1186/gb-2002-3-7-research0034
Vargas-Albores F, Yépiz-Plascencia G, Jiménez-Vega F, Ávila-Villa A (2004) Structural and functional differences of Litopenaeus vannamei crustins. Comparative Biochemistry and Physiology B 138:415–422. https://doi.org/10.1016/j.cbpc.2004.05.007
Vaseeharan B, Ramasamy P (2003) Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Letters in Applied Microbiology 36:83–87. https://doi.org/10.1046/j.1472-765X.2003.01255.x
Vaseeharan B, Shanthi S, Chen JC, Espiñeira M (2012) Molecular cloning, sequence analysis and expression of Fein-Penaeidin from the hemocytes of Indian white shrimp Fenneropenaeus indicus. Results in Immunology 2:35–43. https://doi.org/10.1016/j.rinim.2012.02.001
Vinoj G, Vaseeharan B, Thomas S, Spiers AJ, Shanthi S (2014) Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Marine Biotechnology 16:707–715. https://doi.org/10.1007/s10126-014-9585-9
Visetnan S, Supungul P, Tassanakajon A, Donpudsa S, Rimphanitchayakit V (2017) A single WAP domain-containing protein from Litopenaeus vannamei possesses antiproteinase activity against subtilisin and antimicrobial activity against AHPND-inducing Vibrio parahaemolyticus. Fish & Shellfish Immunology 68:341–348. https://doi.org/10.1016/j.fsi.2017.07.046
Wang Y, Tseng CW, Lin HY, Chen I, Chen YH, Chen YM, Chen TY, Yang HL (2010) RNAi knock-down of the Litopenaeus vannamei Toll gene (LvToll) significantly increases mortality and reduces bacterial clearance after challenge with Vibrio harveyi. Developmental & Comparative Immunology 34:49–58. https://doi.org/10.1016/j.dci.2009.08.003
Wu Q, Zhang R, Peng S, Xu Y (2015) Transcriptional characteristics associated with lichenysin biosynthesis in Bacillus licheniformis from Chinese Maotai-flavor liquor making. Journal of Agricultural & Food Chemistry 63:888–893. https://doi.org/10.1021/jf5036806
Xie JJ, Liu QQ, Liao S, Fang HH, Yin P, Xie SW, Niu J (2019) Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology 90:456–465. https://doi.org/10.1016/j.fsi.2019.04.301
Zokaeifar H, Saad CRB, Daud HM, Harmin SA, Shakibazadeh S (2009) Effect of Bacillus subtilis on the growth and survival rate of shrimp (Litopenaeus vannamei). African Journal of Biotechnology 8:3369–3376
Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, Nejat N (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology 33:683–689. https://doi.org/10.1016/j.fsi.2012.05.027
Zokaeifar H, Babaei N, Saad CR, Kamarudin MS, Sijam K, Balcazar JL (2014) Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish & Shellfish Immunology 36:68–74. https://doi.org/10.1016/j.fsi.2013.10.007
Acknowledgments
Dario I. García Medel thanks the Instituto Tecnológico de Boca del Río (16990169) for academic support and D. Fischer for editorial services.
Funding
This study was financed by the Secretaria de Investigacion y Posgrado del Instituto Politecnico Nacional (Mexico) and Consejo Nacional de Ciencia y Tecnologiia (20181360 to A. L-G and PDCPN2014-01/248033 to CA).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was followed by the authors in accordance with regulations of the international and national guidelines for care and use of animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Bacillus licheniformis BCR 4-3 in water increased survival of shrimp challenged with V. parahaemolyticus-AHPND.
• B. licheniformis BCR 4-3 in diet increased growth performance of shrimp.
• Innate immune-related genes were upregulated in shrimp fed B. licheniformis BCR4-3.
• B. licheniformis BCR4-3 is an immunoprophylactic alternative against AHPND in shrimp Litopenaeus vanammei.
Rights and permissions
About this article
Cite this article
García-Medel, D.I., Angulo, C., Escamilla-Montes, R. et al. Bacillus licheniformis BCR 4-3 increases immune response and survival of Litopenaeus vannamei challenged with Vibrio parahaemolyticus IPNGS16. Aquacult Int 28, 2303–2318 (2020). https://doi.org/10.1007/s10499-020-00585-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00585-2