Abstract
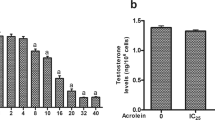
H2O2 is one of the active reactive oxygen species secreted by macrophages that are seen closely aligned with Leydig cells in the testicular interstitium. The present study was initiated to investigate the role of H2O2 on Leydig cell function in vitro at physiological concentrations. Significant decrease in both testosterone production (p < 0.05) and 3 β-hydroxysteroid dehydrogenase activity (p < 0.05) in adult Leydig cells were observed even with H2O2 at low concentrations (30 – 50 μM). H2O2 exposure increased oxidative stress in Leydig cells with the rise in lipid peroxidation and fall in the activities of the antioxidant enzymes; superoxide dismutase (SOD), catalase (CAT) & glutathione-s-transferase (GST). There was also a marginal increase (∼8%) in cell apoptosis accompanied by rise in FasL expression and caspase-3 activation. The above findings indicate that H2O2 as a bio-molecule modulates Leydig cell function at or below physiological concentrations through a variety of actions like decrease in steroidogenic enzyme activity and increase in oxidative stress and apoptosis.
Similar content being viewed by others
References
Chao CM, Jin JS, Tsai CS, et al. Effect of hydrogen peroxide on intracellular pH in the human arterial myocardium. Chin J Physiol 2002; 45: 123–129.
Riley J, Behrman HR. In vivo generation of hydrogen peroxide during luteolysis. Endocrinology 1991; 128: 1749–1753.
Behrman HR, Preston SL. Luteolytic action of peroxides in rat ovarian cells. Endocrinology 1989; 124: 2895–2900.
Margolin Y, Aten RF, Behrman HR. Antigonadotropic and antiestrogenic actions of peroxide in rat granulose cells. Endocrinology 1990; 127: 245–250.
Nunez J, Pommier J. Formation of thyroid hormones. Vitam Horm 1982; 39: 175–229.
Barbouti A, Doulias PT, Nousis L, Tenopoulou M, Galaris D. DNA damage and apoptosis in hydrogen peroxide-exposed jurkat cells: Bolus versus continuous generation of H2O2. Free Radical Biol & Med 2002; 33: 691–702.
Lee BR, Um HD. Hydrogen peroxide suppresses U937 cell death by two different mechanisms depending on its concentration. Exp Cell Res 1999; 248: 430–438.
Richter-Landsberg C, Vollgraf U. Mode of cell injury and death after hydrogen peroxide exposure in oligodendroglia cells. Exp Cell Res 1998; 244: 218–229.
Fawcet DW, Neaves WB, Flres MN. Comparative observations on interstitial lymphatics and organization of the interstitial tissue of the rat testis. Biol Reprod 1973; 9: 500–532.
Kern S, Robertson SA, Mau VJ, Maddocks S. Cytokine secretion by macrophages in the rat testis. Biol Reprod 1995; 1407–1416.
Teerds KJ. The Leydig cell. In: Payne AH, Hardy MP, Russell LD, eds. Vienna: Cache River Press, 1996.
Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol 2002; 57: 3–18.
Allen J, Ginde S, Chol J, Diemer T, Held Hales DB. Reactive oxygen disrupts mitocondria in leydig cells and inhibits steroidogenesis. Biol Reprod 2000; 63: 338–342.
Diemer T, Held HK, Ginde S, et al. Immune activation via injection of bacterial lipopolysaccharide (LPS) in mice results in disruption of leydig cells steroidogenesis due to oxidative mitochondrial damage. Biol Reprod 2000; 63: 343--348.
Cooke BA, Aldred F, Hunter MG, Sullivan MHF, Dix CJ. Effects of isolation and purification on the viability and properties of testis leydig cells. J Steroid Biochem 1983; 19(1): 359–366.
Wei RQ, Yee JB, Straus DC, Huston JC. Bactericidal activity of testicular macrophages. Biol Reprod 1988; 38: 830–835.
Kern S, Robertson SA, Mau VJ, Maddocks S. Cytokine secretion by macrophages in the rat testis. Biol Reprod 1995; 53: 1407–1416.
Sharpe RM, Cooper I. Variation in the steroidogenic responsiveness of isolated rat Leydig cells. J. Reprod. Fert 1982; 65: 475–481.
Aldred LF and Cooke BA. The effects of cell damage on the density and steroidogenic capacity of rat testis leydig cells, using an NADH exclusion test for determination of viability. J Steroid Biochem 1983; 18(4): 411–414.
Abraham GE. Radioimmunoassay of steroids in biological fluids. Clin Biochem 1974; 7(3): 193–201.
Talalay P. Hydroxysteroid dehydrogenase. In: Colowick SP, Kaplan NO3 eds. Methods in Enzymology, New York: Academic Press, 1962; 512.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358.
Das K, Samanta L, Chainy GBN. A modified spectrophotometric assay for superoxide dismutase using nitrite formation by superoxide radicals. IJBB 2000; 37: 201–204.
Aebi H. Catalase. In: Bergrneyer HU, ed. Methods in Enzymatic Analysis, New York: Academic Press, 1974; 2: 673–678.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249: 7130–7139.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
Chaki SP, Ghosh D, Misro MM. Simultaneous increase in germ cell apoptosis and oxidative stress under acute unilateral testicular ischemia in rats. Intl J Androl 2003; 26: 1–10.
Cote SL, Ribeiro-Da-Silva A, Claudio Cuello A. Current protocols for light microscopy immunocytochemistry. In: Cuello AC, ed. Immunohistochemistry II, New York: Wiley Press 1993; 154–165.
Kim EC, Yun BS, Ryoo IJ, et al. Complestatin prevents apoptotic cell death: Inhibition of a mitochondrial caspase pathway through AKT/PKB activation. BBRC 2003; 11: 104.
Tsai SC, Lu CC, Lin CS, Wang PS. Antisteroidogenic actions of hydrogen peroxide on rat Leydig cells. J Cell Biochem 2003; 90(6): 1276–1286.
Kim DK, Cho ES, Um HD. Caspase dependent and independent events in apoptosis induced by hydrogen peroxide. Exp Cell Res 2000; 257: 82–88.
Endo T, Aten RF, Leykin L, Behrman HR. Hydrogen peroxide evokes antisteroidogenic and antigonadotropic actions in human granulose luteal cells. J Clin Endocrinol Metab 1993; 76: 337–342.
Stocco DM, Wells J, Clark BJ. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig tumor cells. Endocrinology 1993; 133(6): 2827–2832.
Berhaman HR, Aten RF. Evidence that hydrogen peroxide blocks hormone sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology 1991; 128: 2958–2966.
Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003; 144(7): 2882–2891.
Purvis K, Clausen OPF, Hansson V. Age related changes in responsiveness of rat leydig cells to hCG. J Reprod Fert 1978; 52: 379–386.
Paz GF, Winter ISD, Reyes FI, Faiman C. Developmental pattern of testosterone production by the rat testis. Steroids 1980; 36(6): 675–688.
Peltola V, Huhtaniemi I, Metsa-ketela, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology 1996; 137: 105–112.
Myers RB, Abney TO. The effects of reduced O2 and antioxidants on steroidogenic capacity of cultured rat leydig cells. J Steroid Biochem 1988; 31: 305–309.
Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fert 1987; 81: 459–469.
Wei T, Ni Y, Chen C, Zhao B, Xin W. Hydrogen peroxide induced oxidative damage and apoptosis in cerebral granular cells: Protection by ginkgo biloba extract. Phamal Res 2000; 41(4): 427–433.
Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: Implications for apoptosis. FEBS Lett 1997; 414: 552–556.
Richter-Landsberg C, Vollgraff U. Mode of cell injury and death after hydrogen peroxide exposure in cultured oligodendroglia cells. Exp Cell Res 1998; 244: 218–229.
Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunology Today 1994; 15(1): 7–10.
Cohen GM. Caspases: The executioners of apoptosis. Biochem J 1997; 326: 1–16.
Garcia-Calvo M, Petreson EP, Leiting B, Ruel R, Nicholsen DW, Thoenberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 1998; 273: 32608–32618.
Suzuki A, Enari M, Iguchi T. Effect of neonatal exposure to DES in Fas and Bcl-2 expression in the adult mouse vagina and approach to the DES syndrome. Reprod Toxicol 1996; 10: 465–470.
Nair R, Shaha C. Diethylstilbestrol induces rat spermatogenic cell apoptosis in vivo through increased expression of spermatogenic cell Fas/FasL system. J Biol Chem 2003; 278: 6470–6481.
Taylor MF, de Boer-Brouwer M, Woolveridge I, Teerds KJ, Morris ID. Leydig cell apoptosis after the administration of ethane dimethanesulfonate to the adult male rat is a Fas-mediated process. Endocrinology 1999; 140(8): 3797–3804.
Sakamaki K, Yoshida H, Nishimura Y, Nishikawa SI, Manbae N, Yonehare S. Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol Reprod Dev 1997; 47: 11–18.
Hakuno N, Koji T, Yano T, et al. Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicular atresia. Endocrinology 1996; 137: 1938–1948.
Zheng TS, Schlosser SF, Dao T, et al. Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA 1998; 95: 13618–13623.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gautam, D.K., Misro, M.M., Chaki, S.P. et al. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis. Apoptosis 11, 39–46 (2006). https://doi.org/10.1007/s10495-005-3087-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-3087-1