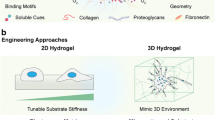
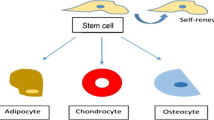
Abstract
Stem cell regenerative potential owing to the capacity to self-renew as well as differentiate into other cell types is a promising avenue in regenerative medicine. Stem cell niche not only provides physical scaffolding but also possess instructional capacity as it provides a milieu of biophysical and biochemical cues. Extracellular matrix (ECM) has been identified as a major dictator of stem cell lineage, thus understanding the structure of in vivo ECM pertaining to specific tissue differentiation will aid in devising in vitro strategies to improve the differentiation efficiency. In this review, we summarize details about the native architecture, composition and mechanical properties of in vivo ECM of the early embryonic stages and the later adult stages. Native ECM from adult tissues categorized on their origin from respective germ layers are discussed while engineering techniques employed to facilitate differentiation of stem cells into particular lineages are noted. Overall, we emphasize that in vitro strategies need to integrate tissue specific ECM biophysical cues for developing accurate artificial environments for optimizing stem cell differentiation.



Similar content being viewed by others
References
Abuhattum, S., and D. Weihs. Integrative biology ratio of total traction force to projected cell area is preserved in differentiating adipocytes. Integr. Biol. 7:1212–1217, 2015.
Agmon, G., and K. L. Christman. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid State Mater. Sci. 20:193–201, 2016.
Agudelo-Garcia, P. A., et al. Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia 13:831-IN22, 2011.
Alfieri, M., et al. A targeted mass spectrometry method to screen collagen types I–V in the decellularized 3D extracellular matrix of the adult male rat thyroid. Talanta 193:1–8, 2019.
Alford, A. I., K. M. Kozloff, and K. D. Hankenson. Extracellular matrix networks in bone remodeling. Int. J. Biochem. Cell Biol. 65:20–31, 2015.
Angammana, C. J., and S. H. Jayaram. The effects of electric field on the multijet electrospinning process and fiber morphology. IEEE Trans. Ind. Appl. 47:1028–1035, 2011.
Badrossamay, M. R., H. A. McIlwee, J. A. Goss, and K. K. Parker. Nanofiber assembly by rotary jet-spinning. Nano Lett. 10:2257–2261, 2010.
Badrossamay, M. R., et al. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials 35:3188–3197, 2014.
Badylak, S. F., D. A. Taylor, and K. Uygun. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13:27–33, 2011.
Baiocchini, A., et al. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS ONE 11:1–14, 2016.
Bakhru, S., et al. Direct and cell signaling-based, geometry-induced neuronal differentiation of neural stem cells. Integr. Biol. 3:1207–1214, 2011.
Ban, D. X., Y. Liu, T. W. Cao, S. J. Gao, and S. Q. Feng. The preparation of rat’s acellular spinal cord scaffold and co-culture with rat’s spinal cord neuron in vitro. Spinal Cord 55:411–418, 2017.
Baptista, P. M., et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53:604–617, 2011.
Barber, J. G., A. M. Handorf, T. J. Allee, and W.-J. Li. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng. A 19:1265–1274, 2011.
Baumann, K. Cell migration: switching to 3D. Nat. Rev. Mol. Cell Biol. 13:338–339, 2012.
Benninger, Y., et al. β1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26:7665–7673, 2006.
Bilston, L. E., and L. E. Thibault. The mechanical properties of the human cervical spinal cord in vitro. Ann. Biomed. Eng. 24:67–74, 1995.
Bonandrini, B., et al. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng. A 20:1486–1498, 2014.
Boon, C. H., T. Cao, L. W. Stanton, P. Robson, and B. Olsen. Strategies for directing the differentiation of stem cells into the osteogenic lineage in vitro. J. Bone Miner. Res. 19:1379–1394, 2004.
Booth, A. J., et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 186:866–876, 2012.
Brandan, E., and N. C. Inestrosa. Isolation of the heparan sulfate proteoglycans from the extracellular matrix of rat skeletal muscle. J. Neurobiol. 18:271–282, 1987.
Brazile, B., et al. Ultrastructure and biomechanics of skeletal muscle ECM: implications in tissue regeneration. In: Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine, edited by J. L. Brown, S. G. Kumbar, and B. L. Banik. Amsterdam: Elsevier, 2016, pp. 139–160.
Bružauskaitė, I., D. Bironaitė, E. Bagdonas, and E. Bernotienė. Scaffolds and cells for tissue regeneration: different scaffold pore sizes: different cell effects. Cytotechnology 68:355–369, 2016.
Burdick, J. A., and G. Vunjak-Novakovic. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A 15:205–219, 2009.
Burgess, J. K., T. Mauad, G. Tjin, J. C. Karlsson, and G. Westergren-Thorsson. The extracellular matrix: the under-recognized element in lung disease? J. Pathol. 240:397–409, 2016.
Burridge, K., and C. Guilluy. Focal adhesions, stress fibers and mechanical tension. Exp. Cell Res. 343:14–20, 2016.
Caló, E., and V. V. Khutoryanskiy. Biomedical applications of hydrogels: a review of patents and commercial products. Eur. Polym. J. 65:252–267, 2015.
Cardwell, R. D., L. A. Dahlgren, and A. S. Goldstein. Electrospun fibre diameter, not alignment, affects mesenchymal stem cell differentiation into the tendon/ligament lineage. J. Tissue Eng. Regen. Med. 8:937–945, 2014.
Case, L. B., and C. M. Waterman. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 17:955–963, 2015.
Chainani, A., et al. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. A 19:2594–2604, 2013.
Chang, C. W., et al. Mesenchymal stem cell seeding of porcine small intestinal submucosal extracellular matrix for cardiovascular applications. PLoS ONE 11:1–19, 2016.
Chani, B., V. Puri, R. C. Sobti, V. Jha, and S. Puri. Decellularized scaffold of cryopreserved rat kidney retains its recellularization potential. PLoS ONE 12:1–20, 2017.
Chanpimol, S., B. Seamon, H. Hernandez, M. Harris-love, and M. R. Blackman. The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen-GAG scaffolds. J. Mech. Behav. Biomed. Mater. 65:295–305, 2017.
Chen, Y.-C., et al. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng. C 21:971–986, 2015.
Choi, Y. C., et al. Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering. Tissue Eng. C 18:866–876, 2012.
Citro, A., et al. The human pancreas as a source of pro-tolerogenic extracellular matrix scaffold for a new generation bio-artificial endocrine pancreas. Ann. Surg. 264:169–179, 2016.
Comley, K., and N. A. Fleck. A micromechanical model for the Young’s modulus of adipose tissue. Int. J. Solids Struct. 47:2982–2990, 2010.
Conti, M. A., S. Even-Ram, C. Liu, K. M. Yamada, and R. S. Adelstein. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 279:41263–41266, 2004.
Cortiella, J., et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. A 16:2565–2580, 2010.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243, 2011.
Daly, A. B., et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng. A 18:1–16, 2011.
Dang, J. M., and K. W. Leong. Myogenic induction of aligned mesenchymal stem cell sheets by culture on thermally responsive electrospun nanofibers. Adv. Mater. 19:2775–2779, 2007.
Dankers, P. Y. W., et al. The use of fibrous, supramolecular membranes and human tubular cells for renal epithelial tissue engineering: towards a suitable membrane for a bioartificial kidney. Macromol. Biosci. 10:1345–1354, 2010.
De Waele, J., et al. 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials 41:122–131, 2015.
DeQuach, J. A., S. H. Yuan, L. S. B. Goldstein, and K. L. Christman. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng. A 17:2583–2592, 2011.
DeQuach, J. A., et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 5:e13039, 2010.
Dong, J., M. Yu, Y. Zhang, Y. Yin, and W. Tian. Recent developments and clinical potential on decellularized adipose tissue. J. Biomed. Mater. Res. A 106:2563–2574, 2018.
Dong, S.-W., et al. Bone regeneration using an acellular extracellular matrix and bone marrow mesenchymal stem cells expressing Cbfa1. Biosci. Biotechnol. Biochem. 73:2226–2233, 2009.
Du, J., et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. 108:9466–9471, 2011.
Duan, Y., et al. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J. Cardiovasc. Transl. Res. 4:605, 2011.
Duband, J.-L., T. Darribère, J.-C. Boucaut, H. Boulekbache, and J. P. Thiery. Regulation of development by the extracellular matrix. Cell Membr. 1198:1–53, 1987.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689, 2006.
Evans, N. D., et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cells Mater. 18:1–13, 2009.
Fan, M., J. Xian, G. Zhou, C. Liang, and S. Liu. Study in vitro on p16beta interfering the cell cycle signal conduction of laryngeal squamous cell carcinoma. Tissue Eng. A 16:515–519, 2010.
Fisher, O. Z., A. L. I. Khademhosseini, R. Langer, and N. A. Peppas. Bioinspired materials for controlling stem cell fate. Acc Chem Res 43:419–428, 2010.
Flanagan, L. A., Y.-E. Ju, B. Marg, M. Osterfield, and P. A. Janmey. Neurite branching on deformable substrates. Neuroreport 13:2411, 2002.
Forte, G., et al. Criticality of the biological and physical stimuli array inducing resident cardiac stem cell determination. Stem Cells 26:2093–2103, 2008.
Frantz, C., K. M. Stewart, and V. M. Weaver. The extracellular matrix at a glance. J. Cell Sci. 123:4195–4200, 2010.
Freedman, B. S., and B. Ratner. Building scaffolds to rebuild kidneys. ACS Cent. Sci. 5:380–382, 2019.
French, K. M., et al. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 8:4357–4364, 2012.
Friedl, P., E. Sahai, S. Weiss, and K. M. Yamada. New dimensions in cell migration. Nat. Rev. Mol. Cell Biol. 13:743–747, 2012.
Fromstein, J. D., et al. Seeding bioreactor-produced embryonic stem cell-derived cardiomyocytes on different porous, degradable, polyurethane scaffolds reveals the effect of scaffold architecture on cell morphology. Tissue Eng. A 14:369–378, 2008.
Fu, R. H., et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 23:621–630, 2014.
Gao, M., et al. Comparative evaluation of decellularized porcine liver matrices crosslinked with different chemical and natural crosslinking agents. Xenotransplantation 26:e12470, 2019.
Garcés-Schröder, M., et al. Characterization of skeletal muscle passive mechanical properties by novel micro-force sensor and tissue micro-dissection by femtosecond laser ablation. Microelectron. Eng. 192:70–76, 2018.
Gershlak, J. R., et al. Mesenchymal stem cells ability to generate traction stress in response to substrate stiffness is modulated by the changing extracellular matrix composition of the heart during development. Biochem. Biophys. Res. Commun. 439:161–166, 2013.
Gilbert, T. W., T. L. Sellaro, and S. F. Badylak. Decellularization of tissues and organs. Biomaterials 27:3675–3683, 2006.
Gillies, A. R., and R. L. Lieber. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44:318–331, 2011.
Gilpin, S. E., et al. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann. Thorac. Surg. 98:1721–1729, 2014.
Goh, S. K., et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34:6760–6772, 2013.
Gong, T., et al. Nanomaterials and bone regeneration. Bone Res. 3:15029, 2015.
Granstrom, L., G. Ekman, U. Ulmsten, and A. Malmstrom. Changes in the connective tissue of corpus and cervix uteri during ripening and labour in term pregnancy. BJOG Int. J. Obstet. Gynaecol. 96:1198–1202, 1989.
Grikscheit, T., and P. De Coppi. Intestinal regeneration. In: Current Concepts of Intestinal Failure, edited by R. J. Rintala, M. Pakarinen, and T. Wester. New York: Springer, 2016, pp. 141–149.
Gritsenko, P. G., O. Ilina, and P. Friedl. Interstitial guidance of cancer invasion. J. Pathol. 226:185–199, 2012.
Guan, Y., et al. Porcine kidneys as a source of ECM scaffold for kidney regeneration. Mater. Sci. Eng. C 56:451–456, 2015.
Haggerty, A. E., M. M. Marlow, and M. Oudega. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci. Lett. 652:50–55, 2017.
Hall, A., et al. Nanonet force microscopy for measuring forces in single smooth muscle cells of the human aorta. Mol. Biol. Cell 28:1894–1900, 2017.
Harada, S. I., and G. A. Rodan. Control of osteoblast function and regulation of bone mass. Nature 423:349–355, 2003.
Hashemi, J., et al. Decellularized pancreas matrix scaffolds for tissue engineering using ductal or arterial catheterization. Cells Tissues Organs 205:72–84, 2018.
He, J. H., Y. Liu, L. Xu, J. Y. Yu, and G. Sun. BioMimic fabrication of electrospun nanofibers with high-throughput. Chaos Solitons Fractals 37:643–651, 2008.
Heydarkhan-Hagvall, S., et al. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 29:2907–2914, 2008.
Higuchi, A., Q. D. Ling, Y. Chang, S. T. Hsu, and A. Umezawa. Physical cues of biomaterials guide stem cell differentiation fate. Chem. Rev. 113:3297–3328, 2013.
Hjelm, A., G. Ekman-Ordeberg, K. Barchan, and A. Malmström. Identification of the major proteoglycans from human myometrium. Acta Obstet. Gynecol. Scand. 80:1084–1090, 2001.
Ho, T., et al. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing e thawing method to investigate stem cell differentiation behaviors. Biomaterials 40:51–60, 2015.
Hollenstein, M., A. Nava, D. Valtorta, J. G. Snedeker, and E. Mazza. Mechanical Characterization of the Liver Capsule and Parenchyma Marc. Lecture Notes in Computer Science. Berlin: Springer, 2006. https://doi.org/10.1016/j.arr.2010.02.003.
Hoshiba, T., H. Lu, N. Kawazoe, and G. Chen. Decellularized matrices for tissue engineering. Expert Opin. Biol. Ther. 10:1717–1728, 2010.
Hoshiba, T., et al. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016. https://doi.org/10.1155/2016/6397820.
Hussein, K. H., K. M. Park, K. S. Kang, and H. M. Woo. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 67:766–778, 2016.
Ihida-Stansbury, K., et al. Role played by Prx1-dependent extracellular matrix properties in vascular smooth muscle development in embryonic lungs. Pulm. Circ. 5:382–397, 2015.
Järveläinen, H. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 61:198–223, 2009.
Kajbafzadeh, A.-M., N. Javan-Farazmand, M. Monajemzadeh, and A. Baghayee. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng. C 19:642–651, 2013.
Kang, X., et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials 28:450–458, 2007.
Keely, P., and A. Nain. Capturing relevant extracellular matrices for investigating cell migration. F1000 Res. 4:1408, 2015.
Ker, E. D. F., et al. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 32:8097–8107, 2011.
Khademhosseini, A., and R. Langer. Microengineered hydrogels for tissue engineering. Biomaterials 28:5087–5092, 2007.
Khetan, S., et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12:458–465, 2013.
Kilian, K. A., B. Bugarija, B. T. Lahn, and M. Mrksich. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. 107:4872–4877, 2010.
Kim, H., M. J. Cooke, and M. S. Schoichet. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol. 30:55–63, 2012.
Kim, G. A., J. R. Spence, and S. Takayama. Bioengineering for intestinal organoid cultures. Curr Opin Biotechnol 47:51–58, 2017.
Klaas, M., et al. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 6:1–12, 2016.
Knippenberg, M., et al. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 11:1780–1788, 2005.
Kohane, D. S., and R. Langer. Polymeric biomaterials in tissue engineering. Pediatr. Res. 63:487–491, 2008.
Koons, B., et al. Cancer protrusions on a tightrope: nanofiber curvature contrast quantitates single protrusion dynamics. ACS Nano 11:12037–12048, 2017.
Koosha, M., and H. Mirzadeh. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. A 103:3081–3093, 2015.
Kopecek, J., and J. Yang. Hydrogels as smart biomaterials. Polym. Int. 1098:1078–1098, 2007.
Koser, D. E., E. Moeendarbary, J. Hanne, S. Kuerten, and K. Franze. CNS cell distribution and axon orientation determine local spinal cord mechanical properties. Biophys. J. 108:2137–2147, 2015.
Lee, J. S., et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 15:206–218, 2013.
Legant, W. R., et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc. Natl. Acad. Sci. 106:10097–10102, 2009.
Leipzig, N. D., and M. S. Shoichet. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30:6867–6878, 2009.
Li, C., C. Vepari, H.-J. Jin, H. J. Kim, and D. L. Kaplan. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 27:3115–3124, 2006.
Li, D., and Y. Xia. Electrospinning of nanofibers: reinventing the wheel? Adv. Mater. 16:1151–1170, 2004.
Liang, R., et al. Positive effects of an extracellular matrix hydrogel on rat anterior cruciate ligament fibroblast proliferation and collagen mRNA expression. J. Orthop. Transl. 3:114–122, 2015.
Lieber, R. L., E. Runesson, F. Einarsson, and J. Fridén. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28:464–471, 2003.
Light, N., and A. E. Champion. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem. J. 219:1017–1026, 1984.
Lim, S. H., and H. Q. Mao. Electrospun scaffolds for stem cell engineering. Adv. Drug Deliv. Rev. 61:1084–1096, 2009.
Liu, Y., D. Luo, and T. Wang. Hierarchical structures of bone and bioinspired bone tissue engineering. Small 12:4611–4632, 2016.
Loneker, A. E., D. M. Faulk, G. S. Hussey, A. D’Amore, and S. F. Badylak. Solubilized liver extracellular matrix maintains primary rat hepatocyte phenotype in-vitro. J. Biomed. Mater. Res. A 104:957–965, 2016.
Lu, Q., M. Li, Y. Zou, and T. Cao. Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novo adipogenesis. J. Control. Release 174:43–50, 2014.
Lu, T., et al. Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials 51:173–183, 2015.
Luo, Y., D. Lou, L. Ma, and C. Gao. Optimizing detergent concentration and processing time to balance the decellularization efficiency and properties of bioprosthetic heart valves. J. Biomed. Mater. Res. A 2019. https://doi.org/10.1002/jbm.a.36732.
Lv, H., et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 6(103):1–11, 2015.
Mahmoud, H., A. Wagoner Johnson, E. K. Chien, M. J. Poellmann, and B. McFarlin. System-level biomechanical approach for the evaluation of term and preterm pregnancy maintenance. J. Biomech. Eng. 135:021009, 2013.
Makino, M., K. Mimatsu, H. Saito, N. Konishi, and Y. Hashizume. Morphometric study of myelinated fibers in human cervical spinal cord white matter. Spine (Phila. Pa. 1976) 21:1010–1016, 1996.
Maldonado, M., et al. Lineage-and developmental stage-specific mechanomodulation of induced pluripotent stem cell differentiation. Stem Cell Res. Ther. 8:1–7, 2017.
McBeath, R., D. M. Pirone, C. M. Nelson, K. Bhadriraju, and C. S. Chen. Cell shape, cytoskeletal tension, and RhoA regulate stemm cell lineage commitment. Dev. Cell 6:483–495, 2004.
McKee, C. T., J. A. Last, P. Russell, and C. J. Murphy. Indentation versus tensile measurements of young’s modulus for soft biological tissues. Tissue Eng. B 17:155–164, 2011.
Meehan, S., and A. S. Nain. Role of suspended fiber structural stiffness and curvature on single-cell migration, nucleus shape, and focal-adhesion-cluster length. Biophys. J. 107:2604–2611, 2015.
Mendez, J. J., M. Ghaedi, D. Steinbacher, and L. E. Niklason. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng. A 20:1735–1746, 2014.
Mirmalek-Sani, S. H., et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials 34:5488–5495, 2013.
Nagao, R. J., et al. Decellularized human kidney cortex hydrogels enhance kidney microvascular endothelial cell maturation and quiescence. Tissue Eng. A 22:1140–1150, 2016.
Nain, A. S., M. Sitti, A. Jacobson, T. Kowalewski, and C. Amon. Dry spinning based spinneret based tunable engineered parameters (STEP) technique for controlled and aligned deposition of polymeric nanofibers. Macromol. Rapid Commun. 30:1406–1412, 2009.
Nain, A. S., and J. Wang. Polymeric nanofibers: isodiametric design space and methodology for depositing aligned nanofiber arrays in single and multiple layers. Polym. J. 45:695–700, 2013.
Nair, L. S., and C. T. Laurencin. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32:762–798, 2007.
Nakayama, K. H., C. C. I. Lee, C. A. Batchelder, and A. F. Tarantal. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS ONE 8:e64134, 2013.
Nam, J., J. Johnson, J. J. Lannutti, and S. Agarwal. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 7:1516–1524, 2011.
Nicodemus, G. D., and S. J. Bryant. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. B 14:149–165, 2008.
O’Neill, J. D., et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 96:1046–1055, 2013.
Oberwallner, B., et al. Human cardiac extracellular matrix supports myocardial lineage commitment of pluripotent stem cells. Eur. J. Cardio-thorac. Surg. 47:416–425, 2015.
Ogle, B. M., S. P. Palecek, D. R. Bogdanowicz, and H. H. Lu. Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol. J. 8:472–484, 2013.
Oh, S. H., I. K. Park, J. M. Kim, and J. H. Lee. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 28:1664–1671, 2007.
Opara, E. C., S.-H. Mirmalek-Sani, O. Khanna, M. L. Moya, and E. M. Brey. Design of a bioartificial pancreas+. J. Investig. Med. 58:831–837, 2010.
Orlando, G., et al. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials 34:5915–5925, 2013.
Ott, H. C., et al. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 14:213–221, 2008.
Ottani, V., et al. Collagen fibril arrangement and size distribution in monkey oral mucosa. J. Anat. 192(Pt 3):321–328, 1998.
Pan, J., et al. Regeneration of a bioengineered thyroid using decellularized thyroid matrix. Thyroid 29:142–152, 2018.
Parmaksiz, M., A. E. Elcin, and Y. M. Elcin. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical-sized full-thickness skin defect, alone and in combination with stem cells, in a small rodent model. J. Tissue Eng. Regen. Med. 11:1754–1765, 2017.
Pati, F., et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5:3935, 2014.
Perniconi, B., et al. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 32:7870–7882, 2011.
Phipps, M. C., et al. Mesenchymal stem cell responses to bone-mimetic electrospun matrices composed of polycaprolactone, collagen I and nanoparticulate hydroxyapatite. PLoS ONE 6:e16813, 2011.
Poh, Y. C., et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 5:1–12, 2014.
Polacheck, W. J., and C. S. Chen. Measuring cell-generated forces: a guide to the available tools. Nat. Methods 13:415–423, 2016.
Puistola, U., L. Risteli, A. Kauppila, M. Knip, and J. Risteli. Markers of type I and type III collagen synthesis in serum as indicators of tissue growth during pregnancy. J. Clin. Endocrinol. Metab. 77:178–182, 1993.
Rafiei, S., S. Maghsoodloo, B. Noroozi, V. Mottaghitalab, and A. K. Haghi. Mathematical modeling in electrospinning process of nanofibers: a detailed review. Cellul. Chem. Technol. 47:323–338, 2013.
Rashtbar, M., et al. Characterization of decellularized ovine small intestine submucosal layer as extracellular matrix-based scaffold for tissue engineering. J. Biomed. Mater. Res. B 106:933–944, 2018.
Reilly, G. C., and A. J. Engler. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 43:55–62, 2010.
Riboldi, S. A., M. Sampaolesi, P. Neuenschwander, G. Cossu, and S. Mantero. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials 26:4606–4615, 2005.
Rivest, C., et al. Microscale hydrogels for medicine and biology: synthesis, characteristics and applications. J. Mech. Mater. Struct. 2:1103–1119, 2007.
Rogalski, J. J., C. W. M. Bastiaansen, and T. Peijs. Rotary jet spinning review: a potential high yield future for polymer nanofibers. Nanocomposites 3:97–121, 2017.
Ross, E. A., et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J. Am. Soc. Nephrol. 20:2338–2347, 2009.
Rozario, T., and D. W. DeSimone. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341:126–140, 2010.
Sackett, S. D., et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 8:1–16, 2018.
Saldin, L. T., M. C. Cramer, S. S. Velankar, L. J. White, and S. F. Badylak. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 49:1–15, 2017.
Schaefer, L., and R. M. Schaefer. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 339:237–246, 2010.
Schenke-Layland, K., et al. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells 26:1537–1546, 2008.
Schmelter, M., B. Ateghang, S. Helmig, M. Wartenberg, and H. Sauer. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 20:1182–1184, 2006.
Sell, S. A., M. J. McClure, K. Garg, P. S. Wolfe, and G. L. Bowlin. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 61:1007–1019, 2009.
Sellaro, T. L., et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. A 16:1075–1082, 2009.
Shabani, I., et al. Enhanced infiltration and biomineralization of stem cells on collagen-grafted three-dimensional nanofibers. Tissue Eng. A 17:1209–1218, 2011.
Sharma, P., et al. Aligned fibers direct collective cell migration to engineer closing and nonclosing wound gaps. Mol. Biol. Cell 28:2579–2588, 2017.
Sheets, K., J. Wang, W. Zhao, R. Kapania, and A. S. Nain. Nanonet force microscopy for measuring cell forces. Biophys. J. 111:197–207, 2016.
Shi, L., and V. Ronfard. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): a mini review. Int. J. Burns Trauma 3:173–179, 2013.
Shin, M., H. Yoshimoto, and J. P. Vacanti. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 10:33–41, 2004.
Shynlova, O., J. A. Mitchell, A. Tsampalieros, B. L. Langille, and S. J. Lye. Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium. Biol. Reprod. 70:986–992, 2004.
Silver, F. H., J. W. Freeman, and G. P. Seehra. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 36:1529–1553, 2003.
Simpson, D. L., et al. Engineering patient-specific valves using stem cells generated from skin biopsy specimens. Ann. Thorac. Surg. 98:947–954, 2014.
Snedeker, J. G., et al. Strain-rate dependent material properties of the porcine and human kidney capsule. J. Biomech. 38:1011–1021, 2005.
Stevens, M. M. Biomaterials for bone Materials that enhance bone regeneration have a wealth of potential. Mater. Today 11:18–25, 2008.
Sunyer, R., A. J. Jin, R. Nossal, and D. L. Sackett. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS ONE 7:1–9, 2012.
Toda, S., N. Koike, and H. Sugihara. Thyrocyte integration, and thyroid folliculogenesis and tissue regeneration: perspective for thyroid tissue engineering. Pathol. Int. 51:403–417, 2001.
Toshima, M., Y. Ohtani, and O. Ohtani. Three-dimensional architecture of elastin and collagen fiber networks in the human and rat lung. Arch. Histol. Cytol. 67:31–40, 2004.
Totonelli, G., et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 33:3401–3410, 2012.
Totti, S., M. C. Allenby, S. B. Dos Santos, A. Mantalaris, and E. G. Velliou. A 3D bioinspired highly porous polymeric scaffolding system for: in vitro simulation of pancreatic ductal adenocarcinoma. RSC Adv. 8:20928–20940, 2018.
Tse, J. R., and A. J. Engler. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS ONE 6:e15978, 2011.
Vacanti, J. P., and R. Langer. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354:S32–S34, 1999.
van Genderen, A. M., J. Jansen, C. Cheng, T. Vermonden, and R. Masereeuw. Renal tubular- and vascular basement membranes and their mimicry in engineering vascularized kidney tubules. Adv. Healthc. Mater. 7:1–13, 2018.
Vlierberghe, S. Van, P. Dubruel, and E. Schacht. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 12:1387–1408, 2011.
Volper, B. D., et al. Influence of acute and chronic streptozotocin-induced diabetes on the rat tendon extracellular matrix and mechanical properties. Am. J. Physiol. Integr. Comp. Physiol. 309:R1135–R1143, 2015.
Wang, J. H. C. Mechanobiology of tendon. J. Biomech. 39:1563–1582, 2006.
Wang, L., J. A. Johnson, Q. Zhang, and E. K. Beahm. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 9:8921–8931, 2013.
Wang, Y., et al. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials 33:485–493, 2012.
Wren, T. A. L., S. A. Yerby, G. S. Beaupre, and D. R. Carter. Mechanical properties of the human achilles tendon. Clin. Biomech. 16:245–251, 2001.
Yamada, K. M., R. Pankov, and E. Cukierman. Dimensions and dynamics in integrin function. Braz. J. Med. Biol. Res. 36:959–966, 2003.
Yan, J., et al. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J. Biomed. Mater. Res. A 100(A):527–535, 2012.
Yang, G., et al. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34:9295–9306, 2013.
Yim, E. K. F., and M. P. Sheetz. Force-dependent cell signaling in stem cell diff erentiation. Stem Cell Res. Ther. 3:41, 2012.
Young, D. A., Y. S. Choi, A. J. Engler, and K. L. Christman. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 34:8581–8588, 2013.
Young, J. L., and A. J. Engler. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32:1002–1009, 2011.
Young, J. L., A. W. Holle, and J. P. Spatz. Nanoscale and mechanical properties of the physiological cell: ECM microenvironment. Exp. Cell Res. 343:3–6, 2016.
Youngstrom, D. W., J. G. Barrett, R. R. Jose, and D. L. Kaplan. Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS ONE 8:e64151, 2013.
Youngstrom, D. W., I. Rajpar, D. L. Kaplan, and J. G. Barrett. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. J. Orthop. Res. 33:911–918, 2015.
Yuan, N., et al. Neural stem cell transplantation in a double-layer collagen membrane with unequal pore sizes for spinal cord injury repair. Neural Regen. Res. 9:1014–1019, 2014.
Zhang, J., B. Li, and J. H. C. Wang. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials 32:6972–6981, 2011.
Zhang, J., et al. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials 89:114–126, 2016.
Zhou, Y., et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 73:77–104, 2018.
Zoldan, J., et al. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials 32:9612–9621, 2011.
Acknowledgments
Authors gratefully acknowledge the support provided by Institute for Critical Technology and Applied Science ICTAS), Virginia Tech. This research was partially funded NSF Grants CMMI-1437101 and CMMI-1462916 to ASN.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Emmanuel Opara oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Padhi, A., Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann Biomed Eng 48, 1071–1089 (2020). https://doi.org/10.1007/s10439-019-02337-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02337-7