Abstract
Introduction
This study investigated the therapeutic effect of dual-frequency sonication (3 MHz and 28 kHz) at low intensity levels in combination with micellar doxorubicin in the treatment of a tumor model of spontaneous breast adenocarcinoma in Balb/c mice.
Methods
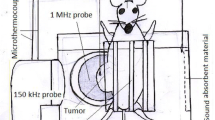
We used sonication frequencies 28 kHz and 3 MHz and their dual combinations in the progressive wave mode to enhance acoustic cavitation. Then, the antitumor effect of the simultaneous dual-frequency ultrasound (28 kHz and 3 MHz) at low intensity levels in combination with doxorubicin and micellar doxorubicin injection was investigated in a spontaneous model of breast adenocarcinoma in Balb/c mice. Sixty-three tumor-bearing mice were randomly divided into seven groups: control, sham, sonication with dual frequency, doxorubicin without sonication, doxorubicin with dual-frequency sonication, micellar doxorubicin without sonication, and micellar doxorubicin with dual-frequency sonication. The tumor volume change relative to the initial volume, tumor growth inhibition ratio, the required times for each tumor to reach two (T 2) and five (T 5) times its initial volume, and survival period were the tumor growth delay parameters which were calculated and recorded at various times after treatment.
Results
The results of the combination of frequencies 28 kHz (0.04 W/cm2) and 3 MHz (2.00 W/cm2) showed remarkable enhancement of the cavitation activity compared with single-frequency sonication (P < 0.05). The micellar doxorubicin injection with sonication group showed a significant difference in the relative volume percent parameter compared with the other groups (P < 0.05). Additionally, the T 2 and T 5 times in the micellar doxorubicin with sonication group were significantly higher than in the other groups (P < 0.05). Also, the survival period of the mice in the micellar doxorubicin with sonication group was significantly longer than in the other groups (P < 0.05). These findings were verified histopathologically.
Conclusion
This study shows that simultaneous combined dual-frequency ultrasound sonication in continuous mode is effective in producing cavitation activity at low intensity. We conclude that dual-frequency sonication with micellar doxorubicin injection extends survival in a murine breast adenocarcinoma model.











Similar content being viewed by others
References
Barati AH, Mokhtari-Dizaji M, Mozdarani H, et al. Treatment of murine tumors using dual-frequency ultrasound in an experimental in vivo model. Ultrasound Med Biol. 2009;35:756–63.
Gao Z, Fain H, Rapoporrt N. Controlled and targeted tumor chemotherapy by micellar encapsulated drug and ultrasound. J Control Release. 2005;102:203–22.
Yu T, Wang Z, Mason TJ. A review of research into the uses of low level ultrasound in cancer therapy. Ultrason Sonochem. 2004;11:95–103.
Leighton TG. The acoustic bubble. London: Academic Press; 1994. p. 67–93.
Bailey M, Halaas D, Reed J, et al. Dual frequency high intensity focused ultrasound to control bubbles. In: Proceedings of the 2nd international symposium on therapeutic ultrasound. 2002. p. 51.
Barati AH, Mokhtari-Dizaji M, Mozdarani H, et al. Effect of exposure parameters on cavitation induced by low level dual frequency ultrasound. Ultrason Sonochem. 2007;14:783–9.
Feng R, Zhao Y, Zhu C, et al. Enhancement of ultrasonic cavitation yield by multi-frequency sonication. Ultrason Sonochem. 2002;9:231–6.
Hasanzadeh H, Mokhtari-Dizaji M, Bathaie SZ, et al. Enhancement and control of acoustic cavitation yield by low-level dual frequency sonication: a subharmonic analysis. Ultrason Sonochem. 2011;18:394–400.
Husseini G, Myrup G, Pitt W, et al. Factors affecting acoustically triggered release of drugs from polymeric micelles. J Control Release. 2000;69:43–52.
Husseini G, Christensen D, Rapoport N, et al. Ultrasonic release of Doxorubicin from Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide. J Control Release. 2002;83:303–5.
Husseini G, de la Rosa MA, Gabuji T, et al. Release of Doxorubicine from unstablized and stabilized micelles under the action of ultrasound. J Nanosci Nanotechnol. 2007;7:1028–33.
Larina I, Evers B, Ashitkov T, et al. Enhancement of drug delivery in tumors by using interaction of nanoparticles with ultrasound radiation. Technol Cancer Res Treat. 2005;4:217–26.
Marine A, Muniruzzaman M, Rapoport N. Acoustic activation of drug delivery from polymeric micelles: effect of pulsed ultrasound. J Control Release. 2001;71:239–49.
Nelson J, Roeder B, Carmen J, et al. Ultrasonically activated chemotherapeutic drug delivery in a rat model. Cancer Res. 2002;62:7280–3.
Alakhov V, Klinski E, Li S, et al. Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surf B Biointerfaces. 1999;16:113–34.
Colombo PE, Boustta M, Poujol S, et al. Biodistribution of doxorubicin-alkylated poly (l-lysine citramide imide) conjugates in an experimental model of peritoneal carcinomatosis after intraperitoneal administration. Eur J Pharm Sci. 2007;31:43–52.
Fundar A, Cavalli R, Bargoni A, et al. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol Res. 2000;42:337–43.
Kümmerle A, Krueger T, Dusmet M, et al. A validated assay for measuring doxorubicin in biological fluids and tissues in an isolated lung perfusion model: matrix effect and heparin interference strongly influence doxorubicin measurements. J Pharm Biomed Anal. 2003;33:475–94.
Osman AMM, Nemnem MM, Abou-Bakr AA, et al. Effect of methimazole treatment on doxorubicin-induced cardiotoxicity in mice. Food Chem Toxicol. 2009;47:2425–30.
Yokoyama M, Miyauchi M, Yamada N, et al. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res. 1990;50:1693–700.
Yokoyama M, Miyauchi M, Yamada N, et al. Polymer micelles as novel drug carrier: adriamycin-conjugated poly (ethylene glycol)-poly (aspartic acid) block copolymer. J Control Release. 1990;11:269–78.
Kazunori K, Glenn SK, Masayuki Y, et al. Block copolymer micelles as vehicles for drug delivery. J Control Release. 1993;24:119–32.
Miwa A, Ishibe A, Nakano M, et al. Development of novel chitosan derivatives as micellar carriers of taxol. Pharm Res. 1998;15:1844–50.
Son YJ, Jang JS, Cho YW, et al. Biodistribution and anti-tumor efficacy of doxorubicin loaded glycol–chitosan nanoaggregates by EPR effect. J Control Release. 2003;91:135–45.
Yokoyama M, Okano T, Sakurai Y, et al. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–36.
Yoo HS, Park TG. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA–PEG block copolymer. J Control Release. 2001;70:63–70.
Hasanzadeh H, Mokhtari-Dizaji M, Bathaie SZ, et al. Effect of local dual frequency sonication on drug distribution from polymeric nanomicelles. Ultrason Sonochem. 2011;18:1165–71.
Pruitt JD, Husseini G, Rapoport N, et al. Stabilization of Pluronic P-105 micelles with an interpenetrating network of N,N-diethylacrylamide. Macromolecules. 2000;33:9306–9.
Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer: a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–77.
Spiegel MR. Mathematical handbook of formulas and tables. New York: McGraw-Hill; 1990. p. 134–9.
Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–54.
Zhao L, Ma J, Zhai X. Enhanced mechanism of catalytic ozonation by ultrasound with orthogonal dual frequencies for the degradation of nitrobenzene in aqueous solution. Ultrason Sonochem. 2010;17:84–91.
Servant G, Laborde JL, Hita A, et al. On the interaction between ultrasound waves and bubble clouds in mono- and dual-frequency sonoreactors. Ultrason Sonochem. 2003;10:347–55.
Tatake PA, Pandit AB. Modelling and experimental investigation into cavity dynamics and cavitational yield: influence of dual frequency ultrasound sources. Chem Eng Sci. 2002;57:4987–95.
Iernetti G, Ciuti P, Dezhkunov NV, et al. Enhancement of high-frequency acoustic cavitation effects by a low-frequency stimulation. Ultrason Sonochem. 1997;4:263–8.
Ciuti P, Dezkkunov NV, Francescutto A, et al. Study into mechanisms of the enhancement of multibubble sonoluminscence emission in interacting fields of different frequencies. Ultrason Sonochem. 2003;10:337–41.
Carpenedo L, Ciuti P, Francescutto A, et al. Space–time interaction of two ultrasound fields and sonoluminescence during transient cavitation in distilled water. Acoust Lett. 1987;10:178–81.
Kanthale PM, Brotchie A, Ashokkumar M, et al. Experimental and theoretical investigations on sonoluminescence under dual frequency conditions. Ultrason Sonochem. 2008;15:629–35.
Jeong YI, Na HS, Cho KO, et al. Antitumor activity of adriamycin-incorporated polymeric micelles of poly([gamma]-benzyl l-glutamate)/poly(ethylene oxide). Int J Pharm. 2009;365:150–6.
Kwon GS, Naito M, Yokoyama M, et al. Physical entrapment of adriamycin in AB block copolymer micelles. Pharm Res. 1995;12:192–5.
Rapoport N, Pitt WG, Sun H, et al. Drug delivery in polymeric micelles: from in vitro to in vivo. J Control Release. 2003;91:85–95.
Acknowledgments
The authors would like to thank Mr. S. Pour-Beiranvand and Mrs. S. Ebrahimi for the preparation of pathological sections, and Mrs. M. Alamolhoda and Mr. H.R. Miri from Tarbiat Modares University for the animal handling and treatment protocol. We would also like to thank Mr. V. Nilchiani from Pars Nahand Engineering Company for providing assistance in designing the sonication system. This work was supported in part by the Iran National Science Foundation (INSF).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hasanzadeh, H., Mokhtari-Dizaji, M., Zahra Bathaie, S. et al. Dual-frequency ultrasound activation of nanomicellar doxorubicin in targeted tumor chemotherapy. J Med Ultrasonics 41, 139–150 (2014). https://doi.org/10.1007/s10396-013-0484-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-013-0484-x