Abstract
Purpose
To assess the changes in the size of muscle fibers and the composition of myosin heavy chain (MyHC) isoforms in the global layer (GL) and the orbital layer (OL) of rabbit rectus extraocular muscle (EOM) after recession.
Methods
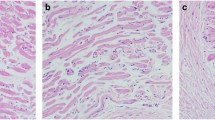
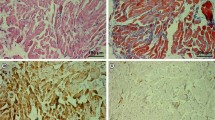
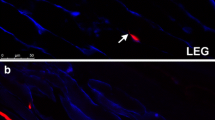
The right superior rectus muscles of two rabbits were harvested at 3 days or 1, 2, or 4 weeks after recession (eight rabbits in total). At each time point, one muscle was used for measuring the cross-sectional area of the muscle fibers and the other for identifying the composition of MyHC. The right superior rectus muscles of three additional naïve rabbits were used as controls.
Results
The mean cross-sectional area of the OL fibers did not change significantly. However, that of the GL fibers significantly decreased at 3 days (P < 0.001) and 1 week (P = 0.024) postoperatively, and increased thereafter to reach the control levels at 2 and 4 weeks postoperatively. Three days after surgery, the total MyHC content and the proportion of type IIb MyHC (MyHCIIb) plus EOM-specific MyHC (MyHCeom) decreased and remained at its lower level for 4 weeks.
Conclusions
Transient atrophy and regeneration were observed only in the GL, and the changes in the MyHCIIb plus MyHCeom appeared to be related to these changes.
Similar content being viewed by others
References
Durston JHJ. Histochemistry of primate extraocular muscles and changes of denervation. Br J Ophthalmol 1974;58:193–216.
Spencer RF, Porter JD. Structural organization of the extraocular muscles. Rev Oculomot Res 1988;2:33–79.
Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol 1995;39:451–484.
Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci 1995;36:1125–1136.
Khanna S, Porter JD. Evidence for rectus extraocular muscle pulleys in rodents. Invest Ophthalmol Vis Sci 2001;42:1986–1992.
Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann N Y Acad Sci 2002;956:17–32.
Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch 2001;443:6–17.
Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 1990;116:1–76.
Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol (Lond) 1991;437:655–672.
Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 1996;76:371–423.
Schiaffiano S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 1994;77:493–501.
Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol 1994;267:C1723–1728.
Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol 1985;101:618–629.
Sartore S, Mascarello F, Rowlerson A, et al. Fibre types in extraocular muscles: a new myosin isoform in the fast fibres. J Muscle Res Cell Motil 1987;8:161–172.
Rubinstein NA, Hoh JF. The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci 2000;41:3391–3398.
Pedrosa-Domellöf F, Holmgren Y, Lucas CA, Hoh JF, Thornell LE. Human extraocular muscles: unique pattern of myosin heavy chain expression during myotube formation. Invest Ophthalmol Vis Sci 2000;41:1608–1616.
Brueckner JK, Itkis O, Porter JD. Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil 1996;17:297–312.
Bormioli SP, Torresan P, Sartore S, Moschini GB, Schiaffino S. Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles. Invest Ophthalmol Vis Sci 1979;18:303–306.
Mascarello F, Rowlerson AM. Myosin isoform transitions during development of extra-ocular and masticatory muscles in the fetal rat. Anat Embryol 1992;185:143–153.
Pedrosa-Domellöf F, Eriksson PO, Butler-Browne GS, Thornell LE. Expression of alpha-cardiac myosin heavy chain in mammalian skeletal muscle. Experientia 1992;48:491–494.
Jakubiec-Puka A, Catani C, Carraro U. Myosin heavy-chain composition in striated muscle after tenotomy. Biochem J 1992;282:237–242.
Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol 1990;69:58–66.
Christiansen SP, Soulsby ME, Seifen EE. Effect of antagonist weakening on developed tension in cat extraocular muscle. Invest Ophthalmol Vis Sci 1995;36:2547–2550.
Christiansen SP, Madhat M, Baker L, Baker R. Fiber hypertrophy in rat extraocular muscle following lateral rectus resection. J Pediatr Ophthalmol Strabismus 1988;25:167–171.
Christiansen SP, McLoon LK. The effect of resection on satellite cell activity in rabbit extraocular muscle. Invest Ophthalmol Vis Sci 2006;47:605–613.
Briggs MM, Schachat F. The superfast extraocular myosin (MYH13) is localized to the innervation zone in both the global and orbital layers of rabbit extraocular muscle. J Exp Biol 2002;205:3133–142.
Asmussen G, Gaunitz U. Changes in mechanical properties of the inferior oblique muscle of the rabbit after denervation. Pflugers Arch 1981;392:198–205.
Kjellgren D, Thornell LE, Anderson J, Pedrosa-Domellöf F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci 2003;44:1419–1425.
Porter JD, Spencer RF. Biological organization of the extraocular muscles. In: Buttner-Ennever J, editor. Progress in brain research: neuroanatomy of the oculomotor system. Amsterdam: Elsevier; 2006. p. 43–80.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Park, S.C., Kim, Y.T., Kim, S.A. et al. Changes in muscle fiber size and in the composition of myosin heavy chain isoforms of rabbit extraocular rectus muscle following recession surgery. Jpn J Ophthalmol 52, 386–392 (2008). https://doi.org/10.1007/s10384-008-0568-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-008-0568-0