Abstract
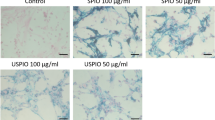
Iron oxide-labelled, single, living human umbilical vein endothelial cells (HUVECs) were imaged over time in vitro using a clinical 3.0-T magnetic resonance (MR) microscopy system. Labelling efficiency, toxicity, cell viability, proliferation and differentiation were assessed using flow cytometry, magnetic cell sorting and a phenanthroline assay. MR images were compared with normal light and fluorescence microscopy. Efficient uptake of iron oxide into HUVECs was shown, although with higher label uptake dose-dependent cytotoxic effects were observed, affecting cell viability. For MR imaging, a T2* weighted three-dimensional protocol was used with in-plane resolution of 39×48μm2 and 100-μm slices with a scan time of 13 min. MRI could detect living cells in standard culture dishes at single-cell resolution, although label loss was observed that corresponded with the intracellular iron measurements. MR microscopy using iron oxide labels is a promising tool for studying HUVEC migration and cell biology in vitro and in vivo, but possible toxic effects of label uptake and loss of label over time should be taken into account.
Similar content being viewed by others
References
Folkman J, Shing Y (1992) Angiogenesis. J Biol Chem 267:10931–10934
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438
Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17:545–580
Bulte JW, Duncan ID, Frank JA (2002) In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab 22:899–907
Neeman M (2000) Preclinical MRI experience in imaging angiogenesis. Cancer Metastasis Rev 19:39–43
Anderson SA, Glod J, Arbab AS, Noel M, Ashari P, Fine HA, Frank JA (2005) Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood 105:420–425
Bulte JW, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17:484–499
Dodd SJ, Williams M, Suhan JP, Williams DS, Koretsky AP, Ho C (1999) Detection of single mammalian cells by high-resolution magnetic resonance imaging. Biophys J 76:103–109
Foster-Gareau P, Heyn C, Alejski A, Rutt BK (2003) Imaging single mammalian cells with a 1.5 T clinical MRI scanner. Magn Reson Med 49:968–971
Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE (2003) Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 102:867–872
Zhang Z, Van den Bos EJ, Wielopolski PA, De Jong-Popijus MA, Duncker DJ, Krestin GP (2004) High resolution magnetic resonance imaging of iron-labeled myoblasts using a standard 1.5 T clinical scanner. MAGMA 3:1–9
Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP (2004) MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA 101:10901–10906
Heyn C, Bowen CV, Rutt BK, Foster PJ (2005) Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med 53:312–320
Shapiro EM, Skrtic S, Koretsky AP (2005) Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med 53:329–338
Jacobs RE, Fraser SE (1994) Magnetic resonance microscopy of embryonic cell lineages and movements. Science 263:681–684
Van den Bos EJ, Wagner A, Mahrholdt H, Thompson RB, Morimoto Y, Sutton BS, Judd RM, Taylor DA (2003) Improved efficacy of stem cell labeling for magnetic resonance imaging studies by the use of cationic liposomes. Cell Transplant 12:743–756
Dunning MD, Lakatos A, Loizou L, Kettunen M, Ffrench-Constant C, Brindle KM, Franklin RJ (2004) Superparamagnetic iron oxide-labeled schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci 24:9799–9810
Bos C, Delmas Y, Desmouliere A, Solanilla A, Hauger O, Grosset C, Dubus I, Ivanovic Z, Rosenbaum J, Charbord P, Combe C, Bulte JW, Moonen CT, Ripoche J, Grenier N (2004) In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology 233:781–789
Cahill KS, Gaidosh G, Huard J, Silver X, Byrne BJ, Walter GA (2004) Noninvasive monitoring and tracking of muscle stem cell transplants. Transplantation 78:1626–1633
Daldrup-Link HE, Rudelius M, Piontek G, Metz S, Brauer R, Debus G, Corot C, Schlegel J, Link TM, Peschel C, Rummeny EJ, Oostendorp RA (2005) Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology 234:197–205
Frank JA, Zywicke H, Jordan EK, Mitchell J, Lewis BK, Miller B, Bryant LH Jr, Bulte JW (2002) Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol 9:S484–S487
Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH Jr, Bulte JW (2003) Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology 228:480–487
Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JW, Frank JA (2003) Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation 76:1123–1130
Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA (2003) Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology 229:838–846
Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, Frank JA (2004) Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging 3:24–32
Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417
Bernardini G, Spinetti G, Ribatti D, Camarda G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M (2000) I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood 96:4039–4045
Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW (2004) Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 17:513–517
Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF (2004) Chondrogenic differentiation of mesenchymal stem cells is inhibited after magnetic labeling with ferumoxides. Blood 104:3410–3412
Haacke EM, Brown RW, Thompson MR, Venkatesan R (1999) Magnetic resonance imaging: physical principles and sequence design. Wiley-Liss, New York
Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA (2004) Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 104:1217–1223
Wardman P, Candeias LP (1996) Fenton chemistry: an introduction. Radiat Res 45:523–531
Chrichton RR, Wilmet S, Legssyer R, Ward RJ (2002) Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem 91:9–19
Legssyer R, Ward RJ, Chrichton RR, Boelaert JR (1999) Effect of chronic chloroquine administration on iron loading in the liver and reticuloendothelial system and on oxidative responses by the alveolar macrophages. Biochem Pharmacol 57:907–911
Unterluggauer H, Hampel B, Zwerschke W, Jansen-Durr P (2003) Senescence-associated cell death of human endothelial cells: the role of oxidative stress. Exp Gerontol 38:1149–1160
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Bos, E., Wielopolski, P. et al. In vitro imaging of single living human umbilical vein endothelial cells with a clinical 3.0-T MRI scanner. MAGMA 18, 175–185 (2005). https://doi.org/10.1007/s10334-005-0108-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-005-0108-6