Abstract
Plant aquaporins are believed to facilitate water transport across cell membranes. However, the relationship between aquaporins and drought resistance in plants remains unclear. VfPIP1, a putative aquaporin gene, was isolated from Vicia faba leaf epidermis, and its expression was induced by abscisic acid (ABA). Our results indicated that the VfPIP1 protein was localized in the plasma membrane, and its expression in V. faba was induced by 20% polyethylene glycol 6000. To further understand the function of VfPIP1, we obtained VfPIP1-expressing transgenic Arabidopsis thaliana plants under the control of the CaMV35S promoter. As compared to the wild-type control plants, the transgenic plants exhibited a faster growth rate, a lower transpiration rate, and greater drought tolerance. In addition, the stomata of the transgenic plants closed significantly faster than those of the control plants under ABA or dark treatment. These results suggest that VfPIP1 expression may improve drought resistance of the transgenic plants by promoting stomatal closure under drought stress.






Similar content being viewed by others
References
Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15:439–447
Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18:565–570
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125:1206–1215
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
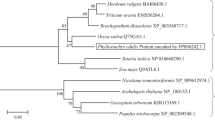
Cui XH, Hao FS, Chen H, Cai JH, Chen J, Wang XC (2005) Isolation and expression of an aquaporin-like gene VfPIP1 in Vicia faba. Prog Nat Sci 15:496–501
Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106:1325–1333
Flexas J, Ribas-Carbo M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48:427–439
Harris MJ, Outlaw Jr WH (1991) Rapid adjustment of guard-cell abscisic acid levels to current leaf-water status. Plant Physiol 95:171–173
Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11:1867–1882
Kaldenhoff R, Kolling A, Meyers J, Karmann U, Ruppel G, Richter G (1995) The blue light-responsive AthH2 gene of Arabidopsis thaliana is primarily expressed in expanding as well as in differentiating cells and encodes a putative channel protein of the plasmalemma. Plant J 7:87–95
Kammerloher W, Fischer U, Piechottka GP, Schaffner AR (1994) Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J 6:187–199
Li L, Li S, Tao Y, Kitagawa Y (2000) Molecular cloning of a novel water channel from rice: its products expression in Xenopus oocytes and involvement in chilling tolerance. Plant Sci 154:43–51
Lian HL, Yu X, Ye Q, Ding XS, Kitagawa Y, Kwak SS, Su WA, Tang ZC (2004) The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 45:481–489
Maurel C, Chrispeels MJ (2001) Aquaporins. A molecular entry into plant water relations. Plant Physiol 125:135–138
McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111:1031–1042
Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R (2002) Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14:727–739
Quigley F, Rosenberg JM, Shachar-Hill Y, Bohnert HJ (2002) From genome to function: the Arabidopsis aquaporins. Genome Biol 3:1–17
Sarda X, Tousch D, Ferrare K, Legrand E, Dupuis JM, Casse-Delbart F, Lamaze T (1997) Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J 12:1103–1111
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52:627–658
Schurr U, Gollan T, Schulze ED (1992) Stomatal response to drying soil in relation to changes in the xylem sap composition of Helianthus annuus. II. Stomatal sensitivity to abscisic acid imported from the xylem sap. Plant Cell Environ 15:561–567
Sheen J (2001) Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol 127:1466–1475
Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14:869–876
Suga S, Komatsu S, Maeshima M (2002) Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol 43:1229–1237
Tsang SS, Yin X, Guzzo-Arkuran C, Jones VS, Davison AJ (1993) Loss of resolution in gel electrophoresis of RNA: a problem associated with the presence of formaldehyde gradients. Biotechniques 14:380–381
Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25:173–194
Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R (2003) The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425:734–737
Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (2004) Novel regulation of aquaporins during osmotic stress. Plant Physiol 135:2318–2329
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (nos. 2006CB100100 and 2003CB114300) and the National Science Foundation of China (nos. 30370129 and 30421002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, XH., Hao, FS., Chen, H. et al. Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J Plant Res 121, 207–214 (2008). https://doi.org/10.1007/s10265-007-0130-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-007-0130-z