Abstract
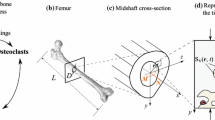
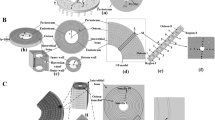
Age-related increases in trabecular bone porosity, as seen in osteoporosis, not only affect the strength and stiffness, but also potentially the mechanobiological response of bone. The mechanical interaction between trabecular bone and bone marrow is one source of mechanobiological signaling, as many cell populations in marrow are mechanosensitive. However, measuring the mechanics of this interaction is difficult, due to the length scales and geometric complexity of trabecular bone. In this study, a multi-scale computational scheme incorporating high-resolution, tissue-level, fluid–structure interaction simulations with discrete cell-level models was applied to characterize the potential effects of trabecular porosity and marrow composition on marrow mechanobiology in human femoral bone. First, four tissue-level models with different volume fractions (BV/TV) were subjected to cyclic compression to determine the continuum level shear stress in the marrow. The calculated stress was applied to three detailed models incorporating individual cells and having differing adipocyte fractions. At the tissue level, compression of the bone along its principal mechanical axis induced shear stress in the marrow ranging from 2.0 to 5.6 Pa, which increased with bone volume fraction and strain rate. The shear stress was amplified at the cell level, with over 90% of non-adipocyte cells experiencing higher shear stress than the applied tissue-level stress. The maximum shear stress decreased by 20% when the adipocyte volume fraction (AVF) increased from 30%, as seen in young healthy marrow, to 45 or 60% AVF typically found in osteoporotic patients. The results suggest that increasing AVF has similar effects on the mechanobiological signaling in bone marrow as decreased volume fraction.






Similar content being viewed by others
References
Aaron JE, Makins NB, Sagreiya K (1987) The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop Relat Res 215:260–271
Bayraktar HH, Keaveny TM (2004) Mechanisms of uniformity of yield strains for trabecular bone. J Biomech 37:1671–1678. doi:10.1016/j.jbiomech.2004.02.045
Birmingham E, Grogan JA, Niebur GL, McNamara LM, McHugh PE (2013) Computational modelling of the mechanics of trabecular bone and marrow using fluid–structure interaction techniques. Ann Biomed Eng 41:814–826. doi:10.1007/s10439-012-0714-1
Birmingham E et al (2015) Mechanical stimulation of bone marrow in situ induces bone formation in trabecular explants. Ann Biomed Eng 43:1036–1050. doi:10.1007/s10439-014-1135-0
Bourne BC, van der Meulen MCH (2004) Finite element models predict cancellous apparent modulus when tissue modulus is scaled from specimen CT-attenuation. J Biomech 37:613–621
Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S (1998) Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res 16:155–162
Bryant JD, David T, Gaskell PH, King S, Lond G (1989) Rheology of bovine bone marrow. Proc Inst Mech Eng H 203:71–75
Campbell JJ, Lee DA, Bader DL (2006) Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology 43:455–470
Castillo AB, Jacobs CR (2010) Mesenchymal Stem Cell Mechanobiology. Curr Osteoporos Rep 8:98–104. doi:10.1007/s11914-010-0015-2
Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR (2016) Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J 30:1504–1511. doi:10.1096/fj.15-276402
Coughlin TR, Niebur GL (2012) Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J Biomech 45:2222–2229
Coughlin TR et al (2016) Primary cilia expression in bone marrow in response to mechanical stimulation in explant bioreactor culture. Eur Cell Mater 32:111–122
Coughlin TR, Voisin M, Schaffler MB, Niebur GL, McNamara LM (2015) Primary cilia exist in a small fraction of cells in trabecular bone and marrow. Calcif Tissue Int 96:65–72. doi:10.1007/s00223-014-9928-6
Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL (1998) Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. Osteoporos Int 8:152–158
Cowin SC (1999) Bone poroelasticity. J Biomech 32:217–238
David V et al (2007) Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148:2553–2562. doi:10.1210/en.2006-1704
Dickerson DA, Sander EA, Nauman EA (2007) Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech Model Mechanobiol 7:191–202. doi:10.1007/s10237-007-0085-y
Dickerson DA, Sander EA, Nauman EA (2008) Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech Model Mechanobiol 7:191–202. doi:10.1007/s10237-007-0085-y
Downey DJ, Simkin PA, Taggart R (1988) The effect of compressive loading on intraosseous pressure in the femoral head in vitro. J Bone Joint Surg Am 70:871–877
Fermor B, Gundle R, Evans M, Emerton M, Pocock A, Murray D (1998) Primary human osteoblast proliferation and prostaglandin E2 release in response to mechanical strain in vitro. Bone 22:637–643
Genetos DC, Geist DJ, Liu DW, Donahue HJ, Duncan RL (2005) Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res 20:41–49. doi:10.1359/Jbmr.041009
Gurkan UA, Akkus O (2008) The mechanical environment of bone marrow: a review. Ann Biomed Eng 36:1978–1991. doi:10.1007/s10439-008-9577-x
Gurkan UA, Gargac J, Akkus O (2010) The sequential production profiles of growth factors and their relations to bone volume in ossifying bone marrow explants. Tissue Eng Part A 16:2295–2306. doi:10.1089/ten.TEA.2009.0565
Gurkan UA, Kishore V, Condon KW, Bellido TM, Akkus O (2011a) A scaffold-free multicellular three-dimensional in vitro model of osteogenesis. Calcif Tissue Int 88:388–401. doi:10.1007/s00223-011-9467-3
Gurkan UA, Krueger A, Akkus O (2011b) Ossifying bone marrow explant culture as a three-dimensional mechanoresponsive in vitro model of osteogenesis. Tissue Eng Part A 17:417–428. doi:10.1089/ten.TEA.2010.0193
Harrigan TP, Jasty M, Mann RW, Harris WH (1988) Limitations of the continuum assumption in cancellous bone. J Biomech 21:269–275
Haudenschild AK, Hsieh AH, Kapila S, Lotz JC (2009) Pressure and distortion regulate human mesenchymal stem cell gene expression. Ann Biomed Eng 37:492–502. doi:10.1007/s10439-008-9629-2
Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P (1999) Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167–1174. doi:10.1359/jbmr.1999.14.7.1167
Huang C, Ogawa R (2012) Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells. Tissue Eng Part A 18:2106–2113. doi:10.1089/ten.TEA.2012.0064
Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22:313–323. doi:10.1634/stemcells.22-3-313
Jaasma MJ, Bayraktar HH, Niebur GL, Keaveny TM (2002) Biomechanical effects of intraspecimen variations in tissue modulus for trabecular bone. J Biomech 35:237–246
Jagodzinski M et al (2008) Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture. J Biomech 41:1885–1891
Jun L et al (2009) Hydrostatic pressures promote initial osteodifferentiation with ERK1/2 not p38 MAPK signaling involved. J Cell Biochem 107:224–232
Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2:165–171
Kapur S, Baylink DJ, Lau KH (2003) Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone 32:241–251
Kelly DJ, Jacobs CR (2010) The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today 90:75–85. doi:10.1002/bdrc.20173
Keyak JH, Fourkas MG, Meagher JM, Skinner HB (1993) Validation of an automated method of three-dimensional finite element modelling of bone. J Biomed Eng 15:505–509
Klein-Nulend J, Semeins CM, Burger EH (1996) Prostaglandin mediated modulation of transforming growth factor-beta metabolism in primary mouse osteoblastic cells in vitro. J Cell Physiol 168:1–7. doi:10.1002/(SICI)1097-4652(199607)168:1<1::AID-JCP1>3.0.CO;2-T
Lane JM, Vigorita VJ (1983) Osteoporosis. J Bone Joint Surg Am 65:274–278
Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT (2009) Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res 24:50–61. doi:10.1359/jbmr.080817
Lynch ME et al (2013) In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J Bone Miner Res 28:2357–2367. doi:10.1002/jbmr.1966
Lynch ME, Fischbach C (2014) Biomechanical forces in the skeleton and their relevance to bone metastasis: biology and engineering considerations. Adv Drug Deliv Rev 79–80:119–134. doi:10.1016/j.addr.2014.08.009
Mantila Roosa SM, Liu Y, Turner CH (2011) Gene expression patterns in bone following mechanical loading. J Bone Miner Res 26:100–112. doi:10.1002/jbmr.193
McAllister TN, Du T, Frangos JA (2000) Fluid shear stress stimulates prostaglandin and nitric oxide release in bone marrow-derived preosteoclast-like cells. Biochem Biophys Res Commun 270:643–648. doi:10.1006/bbrc.2000.2467
McAllister TN, Frangos JA (1999) Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res 14:930–936. doi:10.1359/jbmr.1999.14.6.930
Metzger TA, Shudick JM, Seekell R, Zhu Y, Niebur GL (2014) Rheological behavior of fresh bone marrow and the effects of storage. J Mech Behav Biomed Mater 40:307–313. doi:10.1016/j.jmbbm.2014.09.008
Metzger TA, Schwaner SA, LaNeve AJ, Kreipke TC, Niebur GL (2015) Pressure and shear stress in trabecular bone marrow during whole bone loading. J Biomech 48:3035–3043. doi:10.1016/j.jbiomech.2015.07.028
Metzger TA, Niebur GL (2016) Comparison of solid and fluid constitutive models of bone marrow during trabecular bone compression. J Biomech. doi:10.1016/j.jbiomech.2016.09.018
Meyer LA, Johnson MG, Cullen DM, Vivanco JF, Blank RD, Ploeg HL, Smith EL (2016) Combined exposure to big endothelin-1 and mechanical loading in bovine sternal cores promotes osteogenesis. Bone 85:115–122. doi:10.1016/j.bone.2016.02.001
Nauman EA, Satcher RL, Keaveny TM, Halloran BP, Bikle DD (2001) Osteoblasts respond to pulsatile fluid flow with short-term increases in PGE(2) but no change in mineralization. J Appl Physiol 90:1849–1854
Nelson ER, Habibi HR (2013) Estrogen receptor function and regulation in fish and other vertebrates. Gen Comp Endocrinol 192:15–24. doi:10.1016/j.ygcen.2013.03.032
Niebur GL, Feldstein MJ, Yuen JC, Chen TJ, Keaveny TM (2000) High resolution finite element models with tissue strength asymmetry accurately predict failure of trabecular bone. J Biomech 33:1575–1583
Nishioka S, Fukuda K, Tanaka S (1993) Cyclic stretch increases alkaline phosphatase activity of osteoblast-like cells: a role for prostaglandin E2. Bone Mineral 21:141–150
Ochoa JA, Heck DA, Brandt KD, Hillberry BM (1991) The effect of intertrabecular fluid on femoral head mechanics. J Rheumatol 18:580–584
Qin Y (2003) Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech 36:1427–1437. doi:10.1016/s0021-9290(03)00127-1
Reich KM, Frangos JA (1991) Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol 261:C428–432
Reich KM, McAllister TN, Gudi S, Frangos JA (1997) Activation of G proteins mediates flow-induced prostaglandin E2 production in osteoblasts. Endocrinology 138:1014–1018
Riggs BL, Khosla S, Atkinson EJ, Dunstan CR, Melton LJ III (2003) Evidence that type I osteoporosis results from enhanced responsiveness of bone to estrogen deficiency. Osteoporos Int 14:728–733. doi:10.1007/s00198-003-1437-9
Riggs BL, Spelsberg TC (1989) Mechanisms of estrogen action on bone. Postgrad Med Spec No: 13-14 (discussion 33–43)
Sander EA, Shimko DA, Dee KC, Nauman EA (2003) Examination of continuum and micro-structural properties of human vertebral cancellous bone using combined cellular solid models. Biomech Model Mechanobiol 2:97–107. doi:10.1007/s10237-003-0031-6
Sandino C, McErlain DD, Schipilow J, Boyd SK (2015) The poro-viscoelastic properties of trabecular bone: a micro computed tomography-based finite element study. J Mech behav Biomed Mater 44:1–9. doi:10.1016/j.jmbbm.2014.12.018
Shin CS, Lecanda F, Sheikh S, Weitzmann L, Cheng SL, Civitelli R (2000) Relative abundance of different cadherins defines differentiation of mesenchymal precursors into osteogenic, myogenic, or adipogenic pathways. J Cell Biochem 78:566–577
Smalt R, Mitchell FT, Howard RL, Chambers TJ (1997) Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol 273:E751–758
Soves CP, Miller JD, Begun DL, Taichman RS, Hankenson KD, Goldstein SA (2014) Megakaryocytes are mechanically responsive and influence osteoblast proliferation and differentiation. Bone 66C:111–120. doi:10.1016/j.bone.2014.05.015
Tournaire N, Jaffre C, Jacob M, Ducher G, Benhamou C, Courteix D, Meddahi-Pelle A (2007) MMP2 and MMP9 plasma levels as markers of bone remodeling: a study on young male tennis players. Sci Sports 22:123–125
Vaughan TJ, Voisin M, Niebur GL, McNamara LM (2015) Multiscale modeling of trabecular bone marrow: understanding the micromechanical environment of mesenchymal stem cells during osteoporosis. J Biomech Eng. doi:10.1115/1.4028986
Vivanco J, Garcia S, Ploeg HL, Alvarez G, Cullen D, Smith EL (2013) Apparent elastic modulus of ex vivo trabecular bovine bone increases with dynamic loading. Proc Inst Mech Eng H 227:904–912. doi:10.1177/0954411913486855
Wang X, Liu X, Niebur GL (2004) Preparation of on-axis cylindrical trabecular bone specimens using micro-CT imaging. J Biomech Eng 126:122–125
Webster D, Schulte FA, Lambers FM, Kuhn G, Muller R (2015) Strain energy density gradients in bone marrow predict osteoblast and osteoclast activity: a finite element study. J Biomech 48:866–874. doi:10.1016/j.jbiomech.2014.12.009
Wu Z, Laneve AJ, Niebur GL (2013) In vivo microdamage is an indicator of susceptibility to initiation and propagation of microdamage in human femoral trabecular bone. Bone 55:208–215. doi:10.1016/j.bone.2013.02.019
Yourek G, McCormick SM, Mao JJ, Reilly GC (2010) Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med 5:713–724. doi:10.2217/rme.10.60
Zhong Z, Akkus O (2011) Effects of age and shear rate on the rheological properties of human yellow bone marrow. Biorheology 48:89–97. doi:10.3233/BIR-2011-0587
Acknowledgements
This research was supported by the U.S. National Science Foundation (CMMI 1435467). Samples were collected through support of the U.S. National Institutes of Health Grant AR052008. TAM was supported by the Whitaker International Program. TJV/LMMc: European Research Council 258992 and the Royal Irish Academy Postdoctoral Mobility Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Metzger, T.A., Vaughan, T.J., McNamara, L.M. et al. Altered architecture and cell populations affect bone marrow mechanobiology in the osteoporotic human femur. Biomech Model Mechanobiol 16, 841–850 (2017). https://doi.org/10.1007/s10237-016-0856-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0856-4