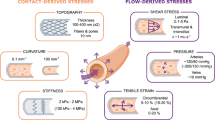
Abstract
In endothelial cells (ECs), the mechanotransduction of fluid shear stress is partially dependent on the transmission of force from the fluid into the cell (mechanotransmission). The role of the primary cilium in EC mechanotransmission is not yet known. To motivate a framework towards quantifying cilia contribution to EC mechanotransmission, we have reviewed mechanical models of both (1) the primary cilium (three-dimensional and lower-dimensional) and (2) whole ECs (finite element, non-finite element, and tensegrity). Both the primary cilia and whole EC models typically incorporate fluid-induced wall shear stress and spatial geometry based on experimentally acquired images of cells. This paper presents future modelling directions as well as the major goals towards integrating primary cilium models into a multi-component EC mechanical model. Finally, we outline how an integrated cilium-EC model can be used to better understand mechanotransduction in the endothelium.


Similar content being viewed by others
References
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM (2009) Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104(7):860–869
Aird WC (2004) Endothelium as an organ system. Crit Care Med 32(5):S271–S279. doi:10.1097/01.CCM.0000129669.21649.40
Ando J, Yamamoto K (2009) Vascular mechanobiology endothelial cell responses to fluid shear stress. Circ J 73(11):1983–1992
Ando J, Yamamoto K (2013) Flow detection and calcium signaling in vascular endothelial cells. Cardiovasc Res 99(2):260–268
Barakat AI (2001) A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells. J Theor Biol 210(2):221–236
Barreto S, Clausen CH, Perrault CM, Fletcher DA, Lacroix D (2013) A multi-structural single cell model of force-induced interactions of cytoskeletal components. Biomaterials 34(26):6119–6126
Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV (2010) Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 20(2):182–187
Buck TE, Li J, Rohde GK, Murphy RF (2012) Toward the virtual cell: automated approaches to building models of subcellular organization “learned” from microscopy images. Bioessays 34(9):791–799
Caille N, Thoumine O, Tardy Y, Meister JJ (2002) Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35(2):177–187
Chouinard JA, Grenier G, Khalil A, Vermette P (2008) Oxidized-LDL induce morphological changes and increase stiffness of endothelial cells. Exp Cell Res 314(16):3007–3016
Costa KD, Yin FCP, Sim AJ (2005) Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. J Biomech Eng 128(2):176–184
Dabagh M, Jalali P, Butler PJ, Trabell JM (2014) Shear-induced force transmission in a multicomponent, multicell model of the endothelium. J R Soc Interface 11(98):20140431. doi:10.1098/rsif.2014.0431
Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garca-Cardea G, Gimbrone MA (2004) Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101(41):14871–14876
Dangaria JH, Butler PJ (2007) Macrorheology and adaptive microrheology of endothelial cells subjected to fluid shear stress. Am J Physiol Cell Physiol 293(5):C1568–C1575
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75(3):519–560
Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6(1):16–26
Davies PF, Shi C, DePaola N, Helmke BP, Polacek DC (2001) Hemodynamics and the focal origin of atherosclerosis. Ann N Y Acad Sci 947(1):7–17
Davies PF, Polacek DC, Shi C, Helmke BP (2002) The convergence of haemodynamics, genomics, and endothelial structure in studies of the focal origin of atherosclerosis. Biorheology 39(3):299–306
Deguchi S, Fukamachi H, Hashimoto K, Iio K, Tsujioka K (2009) Measurement and finite element modeling of the force balance in the vertical section of adhering vascular endothelial cells. J Mech Behav Biomed Mater 2(2):173–185
Downs ME, Nguyen AM, Herzog FA, Hoey DA, Jacobs CR (2014) An experimental and computational analysis of primary cilia deflection under fluid flow. Comput Methods Biomech Biomed Eng 17(1):2–10
Egorova AD, van der Heiden K, Poelmann RE, Hierck BP (2012) Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation 83(2):S56–S61
Espinha LC, Hoey DA, Fernandes PR, Rodrigues HC, Jacobs CR (2014) Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskeleton 71(7):435–445
Farnum CE, Wilsman NJ (2011) Axonemal positioning and orientation in three-dimensional space for primary cilia: What is known, what is assumed, and what needs clarification. Dev Dyn 240(11):2405–2431
Ferko M, Bhatnagar A, Garcia M, Butler P (2007) Finite-element stress analysis of a multicomponent model of sheared and focally-adhered endothelial cells. Ann Biomed Eng 35(2):208–223
Ferko MC, Patterson BW, Butler PJ (2006) High-resolution solid modeling of biological samples imaged with 3d fluorescence microscopy. Microsc Res Tech 69(8):648–655
Frisch-Fay R (1962) Flexible bars. Butterworths, London
Fung YC, Liu SQ (1993) Elementary mechanics of the endothelium of blood vessels. J Biomech Eng 115(1):1–12
Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA (2010) Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466(7303):263–266
Guo P, Weinstein AM, Weinbaum S (2000) A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Ren Physiol 279(4):F698–712
Hagiwara H, Kano A, Aoki T, Ohwada N (2000) Immunocytochemistry of the striated rootlets associated with solitary cilia in human oviductal secretory cells. Histochem Cell Biol 114(3):205–212
Hagiwara H, Ohwada N, Aoki T, Suzuki T, Takata K (2008) The primary cilia of secretory cells in the human oviduct mucosa. Med Mol Morphol 41(4):193–198
Haust MD (1987) Endothelial cilia in human aortic atherosclerotic lesions. Virchows Archiv 410(4):317–326
Helmke BP, Thakker DB, Goldman RD, Davies PF (2001) Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys J 80(1):184–194
Helmke BP, Rosen AB, Davies PF (2003) Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J 84(4):2691–2699
Herzog F (2010) A mechanical approach to study the bending of the primary cilium in response to fluid flow. Masters
Hoey DA, Downs ME, Jacobs CR (2012) The mechanics of the primary cilium: an intricate structure with complex function. J Biomech 45(1):17–26
Ingber DE (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59(1):575–599
Ingber DE (2008) Tensegrity and mechanotransduction. J Bodyw Mov Ther 12(3):198–200
Iomini C, Tejada K, Mo W, Vaananen H, Piperno G (2004) Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 164(6):811–817
Jean RP, Chen CS, Spector AA (2005) Finite-element analysis of the adhesion–cytoskeleton–nucleus mechanotransduction pathway during endothelial cell rounding: axisymmetric model. J Biomech Eng 127(4):594–600
Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS (2004) Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 28(2):101–110
Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE (2006) Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J 90(10):3762–3773
Kwon RY, Hoey DA, Jacobs CR (2011) Mechanobiology of primary cilia cellular and biomolecular mechanics and mechanobiology, studies in mechanobiology, tissue engineering and biomaterials, vol 4. Springer, Berlin
Leiderman KM, Miller LA, Fogelson AL (2008) The effects of spatial inhomogeneities on flow through the endothelial surface layer. J Theor Biol 252:313–325
Lim CT, Zhou EH, Quek ST (2006) Mechanical models for living cellsa review. J Biomech 39(2):195–216
Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM (2003) Effect of flow and stretch on the \([\text{ Ca }^{2+}]_{i}\) response of principal and intercalated cells in cortical collecting duct. Am J Physiol Ren Physiol 285(5):F998–F1012
Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF (2006) Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular ca\(^{2+}\) and camp signaling. Gastroenterology 131(3):911–920
Mathur AB, Truskey GA, Monty Reichert W (2000) Atomic force and total internal reflection fluorescence microscopy for the study of force transmission in endothelial cells. Biophys J 78(4):1725–1735
Mazzag B, Barakat A (2011) The effect of noisy flow on endothelial cell mechanotransduction: a computational study. Ann Biomed Eng 39(2):911–921
McMurray RJ, Wann AKT, Thompson CL, Connelly JT, Knight MM (2013) Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Scientific reports 3
Moser JJ, Fritzler MJ, Ou Y, Rattner JB (2010) The pcmbasal body/primary cilium coalition. Semin Cell Dev Biol 21(2):148–155
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117(9):1161–1171
Park CY, Tambe D, Alencar AM, Trepat X, Zhou EH, Millet E, Butler JP, Fredberg JJ (2010) Mapping the cytoskeletal prestress. Am J Physiol-Cell Physiol 298(5):C1245–C1252
Pitaval A, Tseng Q, Bornens M, Thery M (2010) Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J Cell Biol 191(2):303–312
Poole CA, Zhang ZJ, Ross JM (2001) The differential distribution of acetylated and detyrosinated alpha-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J Anat 199(4):393–405
Pozrikidis C (2010) Shear flow over cylindrical rods attached to a substrate. J Fluids Struct 26(3):393–405
Pozrikidis C (2011) Shear flow past slender elastic rods attached to a plane. Int J Solids Struct 48(1):137–143
Radmacher M (2002) Measuring the elastic properties of living cells by the atomic force microscope. In: Jena BP, Heinrich Hrber JK (eds) Methods in cell biology, vol 68. Academic Press, New York, pp 67–90
Rahimzadeh J, Meng F, Sachs F, Wang J, Verma D, Hua SZ (2011) Real-time observation of flow-induced cytoskeletal stress in living cells. Am J Physiol Cell Physiol 301(3):C646–C652
Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H (2010) Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol-Ren Physiol 298(5):F1096–F1102
Satcher JRL, Gimbrone JMA, Dewey JCF, Bussolari SR (1992) The distribution of fluid forces on model arterial endothelium using computational fluid dynamics. J Biomech Eng 114(3):309–316
Satcher RL Jr, Dewey CF Jr (1996) Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton. Biophys J 71(1):109–118
Schwartz EA, Leonard ML, Bizios R, Bowser SS (1997) Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol-Ren Physiol 272(1):F132–F138
Slomka N, Gefen A (2010) Confocal microscopy-based three-dimensional cell-specific modeling for large deformation analyses in cellular mechanics. J Biomech 43(9):1806–1816
Sugita S, Adachi T, Ueki Y, Sato M (2011) A novel method for measuring tension generated in stress fibers by applying external forces. Biophys J 101(1):53–60
Tritton DJ (1988) Physical fluid dynamics. Clarendon Press, Oxford
Ueki Y, Sakamoto N, Ohashi T, Sato M (2009) Morphological responses of vascular endothelial cells induced by local stretch transmitted through intercellular junctions. Exp Mech 49(1):125–134
Ueki Y, Sakamoto N, Sato M (2010a) Cyclic force applied to fas induces actin recruitment depending on the dynamic loading pattern. Open Biomed Eng J 4:34–129
Ueki Y, Sakamoto N, Sato M (2010b) Direct measurement of shear strain in adherent vascular endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun 394(1):94–99
Ueki Y, Uda Y, Sakamoto N, Sato M (2010c) Measurements of strain on single stress fibers in living endothelial cells induced by fluid shear stress. Biochem Biophys Res Commun 395(3):441–446
Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJBM, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (2008) Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 196(2):542–550
Vargas-Pinto R, Gong H, Vahabikashi A, Johnson M (2013) The effect of the endothelial cell cortex on atomic force microscopy measurements. Biophys J 105(2):300–309
Verma D, Ye N, Meng F, Sachs F, Rahimzadeh J, Hua SZ (2012) Interplay between cytoskeletal stresses and cell adaptation under chronic flow. PLoS One 7(9):e44–167
Vogel V, Sheetz M (2006) Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7(4):265–275
Wang N, Naruse K, Stamenovi D, Fredberg JJ, Mijailovich SM, Toli-Nrrelykke IM, Polte T, Mannix R, Ingber DE (2001) Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci 98(14):7765–7770
Wang Y, Shyy JY, Chien S (2008) Fluorescence proteins, live-cell imaging, and mechanobiology: seeing is believing. Annu Rev Biomed Eng 10:1–38
Yamada H, Mouri N, Nobuhara S (2010) Three-dimensional morphometry of single endothelial cells with substrate stretching and image-based finite element modeling. EURASIP J Adv Signal Proc 2010(1):091–616
Young YN, Downs M, Jacobs CR (2012) Dynamics of the primary cilium in shear flow. Biophys J 103(4):629–639
Zeng D, Juzkiw T, Read AT, Chan DH, Glucksberg M, Ethier CR, Johnson M (2010) Youngs modulus of elasticity of schlemms canal endothelial cells. Biomech Model Mechanobiol 9(1):19–33
Acknowledgments
Yi Chung Lim is supported by a University of Auckland Doctoral Scholarship. This work was supported by a Faculty Research Development Fund Grant (3702516, D.S.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, Y.C., Cooling, M.T. & Long, D.S. Computational models of the primary cilium and endothelial mechanotransmission. Biomech Model Mechanobiol 14, 665–678 (2015). https://doi.org/10.1007/s10237-014-0629-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-014-0629-x