Abstract
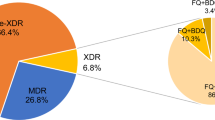
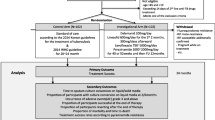
The resistance of Mycobacterium tuberculosis (MTB) to second-line drugs (SLDs) is growing worldwide; however, associations between the appropriateness of treatment for tuberculosis (TB) and whether the directly observed treatment, short course (DOTS)/DOTS-plus programs had an impact on the prevalence of SLD-resistant MTB are still uncertain. We performed a retrospective analysis of resistance profiles among MTB isolates obtained from 6,035 consecutive patients from 2004 to 2011 at two TB referral hospitals in Taiwan. There was a significant decrease (all p-values <0.01) in the prevalence of MTB isolates that were resistant to fluoroquinolones, injectable SLDs, and orally administered SLDs, and multidrug-resistant (MDR) and extensively drug-resistant (XDR) MTB isolates over time. There was a significant increase in the coverage rate of DOTS/DOTS-plus programs and that of administering appropriate first-line and second-line regimens (all p < 0.01). Compared with isoniazid-susceptible isolates, high-level (1.0 mg/L) isoniazid-resistant and MDR isolates showed extensive cross resistance to ofloxacin (5.9 %, p < 0.01 and 33.6 %, p < 0.01), levofloxacin (9.6 %, p < 0.01 and 38.1 %, p < 0.01), moxifloxacin (11.1 %, p < 0.01 and 26.5 %, p < 0.01), kanamycin (6.8 %, p < 0.01 and 16.7 %, p < 0.01), ethionamide (6.4 %, p < 0.01 and 16.2 %, p < 0.01), and para-aminosalicylic acid (13.1 %, p < 0.01 and 20.4 %, p < 0.01), but not to capreomycin (2.0 %, p = 0.06 and 1.6 %, p = 0.08). The decline in prevalence of resistance to SLDs was negatively correlated with the rise in rates of administering appropriate regimens as well as the DOTS/DOTS-plus programs, but not with the increase in usage of second-line regimens. The implementation of DOTS/DOTS-plus programs with appropriate regimens was associated with a decrease in the prevalence of SLD-resistant and XDR TB.


Similar content being viewed by others
References
World Health Organization (WHO) (2011) Global tuberculosis control: WHO report 2011. WHO, Geneva
Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, Fitzmaurice GM, Alcantara Viru FA, Appleton SC, Bayona JN, Bonilla CA, Chalco K, Choi S, Franke MF, Fraser HS, Guerra D, Hurtado RM, Jazayeri D, Joseph K, Llaro K, Mestanza L, Mukherjee JS, Muñoz M, Palacios E, Sanchez E, Sloutsky A, Becerra MC (2008) Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med 359:563–574
Zignol M, Hosseini MS, Wright A, Weezenbeek CL, Nunn P, Watt CJ, Williams BG, Dye C (2006) Global incidence of multidrug-resistant tuberculosis. J Infect Dis 194:479–485
Centers for Disease Control and Prevention (CDC) (2006) Notice to readers: revised definition of extensively drug-resistant tuberculosis. MMWR Morb Mortal Wkly Rep 55:1176
Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, Luh KT, Hsueh PR (2008) Extensively drug-resistant Mycobacterium tuberculosis during a trend of decreasing drug resistance from 2000 through 2006 at a Medical Center in Taiwan. Clin Infect Dis 47:e57–e63
Althomsons SP, Cegielski JP (2012) Impact of second-line drug resistance on tuberculosis treatment outcomes in the United States: MDR-TB is bad enough. Int J Tuberc Lung Dis 16:1331–1334
Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, Hollm-Delgado MG, Keshavjee S, DeRiemer K, Centis R, D’Ambrosio L, Lange CG, Bauer M, Menzies D; Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB (2013) Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 42:156–168
Dalton T, Cegielski P, Akksilp S, Asencios L, Campos Caoili J, Cho SN, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, Kim HJ, Kliiman K, Kurbatova E, Kvasnovsky C, Leimane V, van der Walt M, Via LE, Volchenkov GV, Yagui MA, Kang H; Global PETTS Investigators, Akksilp R, Sitti W, Wattanaamornkiet W, Andreevskaya SN, Chernousova LN, Demikhova OV, Larionova EE, Smirnova TG, Vasilieva IA, Vorobyeva AV, Barry CE 3rd, Cai Y, Shamputa IC, Bayona J, Contreras C, Bonilla C, Jave O, Brand J, Lancaster J, Odendaal R, Chen MP, Diem L, Metchock B, Tan K, Taylor A, Wolfgang M, Cho E, Eum SY, Kwak HK, Lee J, Lee J, Min S, Degtyareva I, Nemtsova ES, Khorosheva T, Kyryanova EV, Egos G, Perez MT, Tupasi T, Hwang SH, Kim CK, Kim SY, Lee HJ, Kuksa L, Norvaisha I, Skenders G, Sture I, Kummik T, Kuznetsova T, Somova T, Levina K, Pariona G, Yale G, Suarez C, Valencia E, Viiklepp P (2012) Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 380:1406–1417
World Health Organization (WHO) (2012) Multidrug-resistant tuberculosis (MDR-TB) 2012 Update. WHO, Geneva
Jaramillo E, Stop TB Initiative (World Health Organization), Great Britain. Dept. for International Development, & United States. Agency for International Development (2008) Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. World Health Organization, Stop TB Department, Geneva
Chien JY, Lai CC, Tan CK, Chien ST, Yu CJ, Hsueh PR (2013) Decline in rates of acquired multidrug-resistant tuberculosis after implementation of the directly observed therapy, short course (DOTS) and DOTS-Plus programmes in Taiwan. J Antimicrob Chemother 68:1910–1916
Murray PR, Baron EJ (2007) Manual of clinical microbiology, 9th edn. ASM Press, Washington
Clinical and Laboratory Standards Institute (CLSI) (2011) Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; Approved standard—second Edition. CLSI document M24-A2. CLSI, Wayne
World Health Organization (WHO), Stop TB Initiative (World Health Organization) (2010) Treatment of tuberculosis: guidelines, 4th edn. WHO, Geneva
van der Werf MJ, Langendam MW, Huitric E, Manissero D (2012) Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J 39:1511–1519
Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, Raviglione M (2012) Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ 90:111–119D
Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, Hoffner S, Drobniewski F, Barrera L, van Soolingen D, Boulabhal F, Paramasivan CN, Kam KM, Mitarai S, Nunn P, Raviglione M; Global Project on Anti-Tuberculosis Drug Resistance Surveillance (2009) Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet 373:1861–1873
Langendam MW, van der Werf MJ, Huitric E, Manissero D (2012) Prevalence of inappropriate tuberculosis treatment regimens: a systematic review. Eur Respir J 39:1012–1020
Chien HP, Yu MC, Ong TF, Lin TP, Luh KT (2000) In vitro activity of rifabutin and rifampin against clinical isolates of Mycobacterium tuberculosis in Taiwan. J Formos Med Assoc 99:408–411
Cavusoglu C, Karaca-Derici Y, Bilgic A (2004) In-vitro activity of rifabutin against rifampicin-resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Clin Microbiol Infect 10:662–665
Dong Y, Xu C, Zhao X, Domagala J, Drlica K (1998) Fluoroquinolone action against mycobacteria: effects of C-8 substituents on growth, survival, and resistance. Antimicrob Agents Chemother 42:2978–2984
Devasia RA, Blackman A, May C, Eden S, Smith T, Hooper N, Maruri F, Stratton C, Shintani A, Sterling TR (2009) Fluoroquinolone resistance in Mycobacterium tuberculosis: an assessment of MGIT 960, MODS and nitrate reductase assay and fluoroquinolone cross-resistance. J Antimicrob Chemother 63:1173–1178
Alangaden GJ, Kreiswirth BN, Aouad A, Khetarpal M, Igno FR, Moghazeh SL, Manavathu EK, Lerner SA (1998) Mechanism of resistance to amikacin and kanamycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother 42:1295–1297
Jugheli L, Bzekalava N, de Rijk P, Fissette K, Portaels F, Rigouts L (2009) High level of cross-resistance between kanamycin, amikacin, and capreomycin among Mycobacterium tuberculosis isolates from Georgia and a close relation with mutations in the rrs gene. Antimicrob Agents Chemother 53:5064–5068
Maus CE, Plikaytis BB, Shinnick TM (2005) Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob Agents Chemother 49:3192–3197
Maus CE, Plikaytis BB, Shinnick TM (2005) Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 49:571–577
Rüsch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S (2006) Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol 44:688–692
Shi D, Li H, Zhao Y, Jia Q, Coulter C, Li L, Zhu G (2012) Extensively drug-resistant tuberculosis, Central China, 2007–2009. Emerg Infect Dis 18:1904–1905
Tan CK, Lai CC, Liao CH, Chou CH, Hsu HL, Huang YT, Hsueh PR (2009) Comparative in vitro activities of the new quinolone nemonoxacin (TG-873870), gemifloxacin and other quinolones against clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother 64:428–429
Tsukamura M (1969) Cross-resistance relationships between capreomycin, kanamycin, and viomycin resistances in tubecle bacilli from paients. Am Rev Respir Dis 99:780–782
Lee H, Cho SN, Bang HE, Lee JH, Bai GH, Kim SJ, Kim JD (2000) Exclusive mutations related to isoniazid and ethionamide resistance among Mycobacterium tuberculosis isolates from Korea. Int J Tuberc Lung Dis 4:441–447
Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, Collins D, de Lisle G, Jacobs WR Jr (1994) inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227–230
Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O’Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society (2003) American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662
Mitchison DA (2000) Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis 4:796–806
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chien, JY., Tsou, CC., Chien, ST. et al. Direct observation therapy with appropriate treatment regimens was associated with a decline in second-line drug-resistant tuberculosis in Taiwan. Eur J Clin Microbiol Infect Dis 33, 941–948 (2014). https://doi.org/10.1007/s10096-013-2030-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-2030-6