Abstract
Objective
We sought to determine whether high-density lipoprotein (HDL) function was altered in gout patients.
Research design and methods
The study included 95 gout patients and 68 healthy controls. The concentrations of interleukin (IL)-1β and IL-9 were measured by ELISA, and indicators such as blood uric acid, liver and kidney function, blood glucose, and blood lipids were detected. To test for the anti-inflammatory and reverse cholesterol transport (RCT) function of HDL, 11 gout patients and 11 healthy controls were randomly selected for the BioVision cholesterol efflux test, which detects the RCT activity of HDL. To assess the anti-inflammatory function of HDL, cells in co-culture with HDL were treated with inflammatory stimuli such as tumor necrosis factor-α (TNF-α), and then, the cells were assayed for the expression of intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1).
Results
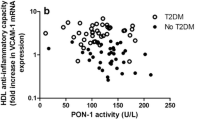
In total, this study enrolled 163 participants, including 95 non-hyperlipidemic gout patients and 68 healthy controls. IL-1β and IL-9 levels were significantly higher in the gout group than in the control group (85.26 ± 23.16 vs. 41.47 ± 6.48 and 33.77 ± 12.68 vs. 23.66 ± 4.53, respectively, P < 0.001). Additionally, plasma IL-1β and IL-9 levels were increased along with those of blood uric acid (R2 = 0.4116 and R2 = 0.4150, respectively, P < 0.001). Compared with the healthy controls, gout patients showed no differences in plasma apoA-1 levels or in the cholesterol efflux assay. Gout patients had increased ICAM-1 expression compared with the healthy controls (88.79 ± 3.68 vs. 86.27 ± 4.64, P < 0.05), but no difference in VCAM-1 expression was found (0.87 ± 0.43 vs. 0.98 ± 0.96, P > 0.05). In this assay, higher values indicate less suppression of ICAM-1 induction, which correlates with a reduced anti-inflammatory capacity.
Conclusions
The anti-inflammatory activities of HDLs are impaired in gout patients.
Key Points • Gout patients show chronic inflammation. • The anti-inflammatory activity of high-density lipoprotein is impaired in gout patients. |




Similar content being viewed by others
References
Barnett R (2018) Gout. Lancet 391(10140):2595
Bevis M, Blagojevic-Bucknall M, Mallen C, Hider S, Roddy E (2018) Comorbidity clusters in people with gout: an observational cohort study with linked medical record review. Rheumatology (Oxford) 57(8):1358–1363
Hsu T-W, Lee P-S, Nfor ON, Lee C-L, Chen P-H, Tantoh DM, Lin L-Y, Chou M-C, Lee Y-C, Liaw Y-P (2019) The interaction between sex and hyperlipidemia on gout risk is modulated by HLA-B polymorphic variants in adult Taiwanese. Genes (Basel) 10(3)
Yü TF, Dorph DJ, Smith H (1978) Hyperlipidemia in primary gout. Semin Arthritis Rheum 7(4):233–244
Elfishawi MM, Zleik N, Kvrgic Z, Michet CJ, Crowson CS, Matteson EL, Bongartz T (2018) The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol 45(4):574–579
Heinecke JW (2009) The HDL proteome: a marker--and perhaps mediator--of coronary artery disease. J Lipid Res 50(Suppl):S167–S171
Wang Y, Wang Z, Li X, Zhang B (2019) Correlation between serum apolipoprotein A1 and serum uric acid level in patients with hyperuricemia. Environ Dis 4(4):95–98
Ouimet M, Barrett TJ, Fisher EA (2019) HDL and reverse cholesterol transport. Circ Res 124(10):1505–1518
Meurs I, Van Eck M, Van Berkel TJC (2010) High-density lipoprotein: key molecule in cholesterol efflux and the prevention of atherosclerosis. Curr Pharm Des 16(13):1445–1467
So AK, Martinon F (2017) Inflammation in gout: mechanisms and therapeutic targets. Nature reviews. Rheumatology 13(11):639–647
Shridas P, De Beer MC, Webb NR (2018) High-density lipoprotein inhibits serum amyloid A-mediated reactive oxygen species generation and NLRP3 inflammasome activation. J Biol Chem 293(34):13257–13269
Thacker SG, Zarzour A, Chen Y, Alcicek MS, Freeman LA, Sviridov DO, Demosky SJ, Remaley AT (2016) High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 149(3):306–319
Scanu A, Luisetto R, Oliviero F, Gruaz L, Sfriso P, Burger D, Punzi L (2015) High-density lipoproteins inhibit urate crystal-induced inflammation in mice. Ann Rheum Dis 74(3):587–594
Hung AM, Tsuchida Y, Nowak KL, Sarkar S, Chonchol M, Whitfield V, Salas N, Dikalova A, Yancey PG, Huang J, Linton MRF, Ikizler TA, Kon V (2019) IL-1 inhibition and function of the HDL-containing fraction of plasma in patients with stages 3 to 5 CKD. Clin J Am Soc Nephrol 14(5):702–711
Bresnihan B, Gogarty M, FitzGerald O, Dayer J-M, Burger D (2004) Apolipoprotein A-I infiltration in rheumatoid arthritis synovial tissue: a control mechanism of cytokine production? Arthritis Res Ther 6(6):R563–R566
Duan L, Huang Y, Qun S, Lin Q, Liu W, Luo J, Yu B, He Y, Qian H, Liu Y, Chen J, Shi G (2016) Potential of IL-33 for preventing the kidney injury via regulating the lipid metabolism in gout patients. J Diabetes Res 2016:102–401
Kristal BS, Vigneau-Callahan KE, Moskowitz AJ, Matson WR (1999) Purine catabolism: links to mitochondrial respiration and antioxidant defenses? Arch Biochem Biophys 370(1):22–33
Lee-Rueckert M, Escola-Gil JC, Kovanen PT (2016) HDL functionality in reverse cholesterol transport - challenges in translating data emerging from mouse models to human disease. Biochim Biophys Acta 1861(7):566–583
Tseng C-C, Chen C-J, Yen J-H, Huang H-Y, Chang J-G, Chang S-J, Liao W-T (2018) Next-generation sequencing profiling of mitochondrial genomes in gout. Arthritis Res Ther 20(1):137
Cardona F, Tinahones FJ, Collantes E, Escudero A, García-Fuentes E, Soriguer FJ (2005) Contribution of polymorphisms in the apolipoprotein AI-CIII-AIV cluster to hyperlipidaemia in patients with gout. Ann Rheum Dis 64(1):85-88
Fazio S, Pamir N (2016) HDL particle size and functional heterogeneity. Circ Res 119(6):704–707
Author information
Authors and Affiliations
Contributions
Yuan Wang and Yan Wang contributed to the study conception and design. Material preparation and data collection were performed by all authors. Data analysis and writing the first draft of the manuscript were performed by Yuan Wang; all authors commented on subsequent versions of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Y., Jia, X. et al. The anti-inflammatory properties of HDLs are impaired in gout. Clin Rheumatol 40, 1525–1531 (2021). https://doi.org/10.1007/s10067-020-05374-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05374-z