Abstract
Objective
Achievement of complete renal remission (CR) is an important goal in lupus nephritis (LN) treatment. The use of cyclosporine (CsA) for active LN has been challenged because of variations in CsA doses and reports of adverse reactions (AR).
Method
A cohort of 62 patients with active LN (induction-resistant LN and flared LN) who were treated with CsA was evaluated. CsA was started at 50 mg/day and titrated up 25 mg/day every 2–4 weeks until CR was achieved or until treatment termination because of AR.
Results
The range of CsA dosage was 50–200 mg/day, and mean CsA dose was 102.8 ± 50.43 mg/day (1.73 ± 0.91 mg/kg/day). CsA plus mycophenolate mofetil and prednisolone was administered to 35.5% of patients, while the other 64.5% were treated with CsA and prednisolone. 90.32% had achieved CR and 4.84% had partial remission after 12 months of treatment. UPCR (urinary protein:creatinine ratio) decreased significantly in both groups (2.58 ± 3.37 to 0.36 ± 0.71 and 2.32 ± 1.45 to 0.29 ± 0.24 respectively) (P < 0.001). Non-renal activity including arthritis, alopecia, hematologic and cutaneous conditions improved in all patients. Patients whose prednisolone dose were increase received higher doses of prednisolone at baseline than patients who had stable prednisolone dose, but after 12 months the difference in dosage was insignificant (p = 0.58).
Conclusion
Patients with active LN can be effectively treated with low dose CsA, and the dose titration approach can lead to 90.32% CR with low AR rates. No difference in clinical response was observed among patients who received CsA plus prednisolone or CsA plus MMF and prednisolone.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) can be a fatal chronic multi-system autoimmune disease. Clinical course of SLE varies depending on whether there is disease flare or remission. Delay in diagnosis and inappropriate treatment may cause vital organ damage and can be life-threatening. The 10-year survival rate of patients with SLE improved from 63% in the year 1950 to 91% in the year 2000, but it then reached a plateau. Renal and neuropsychiatric involvements remain the most important causes of long-term organ damage resulting in poor long-term outcomes [1]. Lupus nephritis (LN) affects more than half of patients with SLE and is a major risk factor of overall mortality in SLE [2]. The current guidelines from the American College of Rheumatology (ACR), and the Joint European League against Rheumatism, and the European Renal Association–European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommend high-dose glucocorticoids in combination with pulse cyclophosphamide or mycophenolate mofetil (MMF) as an initial therapy for LN followed by a maintenance phase with azathioprine, MMF, or a calcineurin inhibitor. Treatment should aim for complete renal remission [3, 4]; however, it is acknowledged that some patients are resistant to initial immunosuppressive therapy and that relapse is very common in proliferative LN patients (5–15 per 100 patient year), and this is particularly true following reduction or cessation of immunosuppressive drugs [5]. In one study, 10 to 30% of patients with LN developed end-stage renal disease [6]. Achieving complete renal remission or complete renal response has a substantial impact on 10-year patient survival, and previous research reported rates of 95% for complete remission, 76% for partial remission, and 19% for no remission [7].
Cyclosporine (CsA) is a cyclic, lipophilic peptide that selectively and reversibly inhibits T cell-mediated immune response by suppressing the phosphatase activity of calcineurin. The inhibitions of IL-2 production and IL-2 receptor expression are the two main stimulating pathways involved in blocking the activation of T cell-specific transcription factors. As a result T cell activation, cytokine production, B cell activation, and immunoglobulin production are reduced [8]. The efficacy of CsA in LN is mediated by its immunosuppressive action, and its antiproteinuric effect acts via its ability to stabilize the podocyte cytoskeleton by inhibiting dephosphorylation and degradation of synaptopodin, an actin-associated protein that regulates cell shape and motility and mediates podocyte foot processes [9]. CsA is currently recommended in the management of LN with persistent severe proteinuria refractory to conventional treatment. A combination of MMF and calcineurin inhibitors has been attracting interest and is suggested by ACR guidelines, but the evidence for this strategy is limited [4, 10]. Furthermore, CsA restores intracellular therapeutic levels of glucocorticoids through the inhibition of P-glycoprotein, which exerts a steroid-sparing effect [11]. CsA has a narrow therapeutic range, and previous studies have revealed that the CsA dose for SLE treatment varies from 1 to 5 mg/kg/day [12]; however, best results were obtained with a daily dose of 3 to 5 mg/kg [13].
To gain a better understanding of the use of CsA in treating active LN and achieving complete renal remission, we evaluated the efficacy and side effects of CsA treatment with and without MMF for patients with active LN.
Patients and methods
A total of 850 SLE patients who fulfilled the American College of Rheumatology (ACR) SLE classification criteria [14] and had been enrolled in our SLE registry since 2006 were reviewed. LN was defined in accordance with ACR criteria [7]. Enrolled subjects were either those who failed to achieve proteinuria level of lower than 1 g/day after a six-month course of standard induction therapy with intravenous cyclophosphamide or MMF (induction-resistant LN), or patients who had LN flare during maintenance therapy (flared LN). All enrolled patients were required to have proteinuria of more than 1 g per day. LN flared was defined as the following: (a) nephritic flare or (b) proteinuric flare. Nephritic flare was defined as a reproducible increase of serum creatinine of ≥ 30% (or a decrease in GFR of ≥ 10%) and active urine sediment increase in glomerular hematuria of ≥ 10 red blood cells per high-power field. Proteinuric flare was defined as a reproducible doubling of UPCR to > 100 mg/mmol (or > 1 g/day) after complete response or reproducible doubling of UPCR to > 200 mg/mmol (or > 2 g per day) after partial response [3]. All patients were treated with CsA micro emulsion and prednisolone for at least 12 months. Patients were classified into two groups according to MMF combination status as CsA and prednisolone group (CsA+P) and CsA plus MMF and prednisolone group (CsA+MMF+P). All patients initially received CsA of 50 mg/day in two divided doses, with slowly titrated doses up 25 mg/day every 2–4 weeks until clinical response had been achieved. The dose of CsA was reduced 25 mg/day from their current dose if serum creatinine had increased by 30% or more from its initial setting. Baseline characteristics were recorded including age, sex, disease duration, previous immunosuppressive drugs, cumulative dose of prednisolone, ACEI/ARB, and antimalarial used and comorbidity conditions. Laboratory investigations including hemoglobin (Hb), total white blood cell count (WBC), lymphocyte count, platelet count (Plt), serum creatinine (Cr), glomerular filtration rate (eGFR), serum albumin (Alb), urine protein creatinine ratio (UPCR), modified SLE disease activity index 2000 (mSLEDAI2K) [15], and non-renal activity and glucocorticoids dosage were also noted at 0, 6 months and 12 months after CSA was initiated. Antimalarial drugs and glucocorticoid dose were administered at the discretion of the physician based on care requirements. Angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) were continued at a constant dose at the time of CsA initiation. Patient who received drugs which interfere with CsA metabolism via cytochrome 3A4 and 3A5 were excluded. At the time of CsA initiation, patients who were treated with increased doses of prednisolone (to more than 20 mg per day) were classified as increased prednisolone group (IncPred) while those who were given the same dose of prednisolone were classified as stable prednisolone group (StaPred). The primary outcome was complete remission (CR), defined as UPCR < 0.5 mg/mmol or proteinuria < 0.5 g/d and normal or near-normal renal function (GFR) [3, 4]. The secondary outcome was partial remission (PR) defined as ≥ 50% reduction in proteinuria to a value of less than 1.5 g per day with a stable serum creatinine [3]. Adverse events from CsA treatment were also recorded. The study was approved by the ethics committee of the investigating hospital.
Statistical analysis
Statistical analysis was performed using SPSS software. Frequency (N, %), mean, and standard deviation (SD) were used for descriptive statistics. Chi-squared test was used to compare categorical data. Paired t test or Wilcoxon Sign Rank test was used to compare the mean of parameters of interest before and after treatment for parametric and non-parametric data as appropriate. Comparison of means between-group was performed using independent t test or Mann–Whitney U test for parameters of interest of parametric and non-parametric samples as appropriate. Comparison of parameters at baseline and 6 and 12 months after cyclosporine treatment were analyzed using repeated measures ANOVA. For all statistical evaluations, p < 0.05 was considered statistically significant.
Results
Patient characteristics
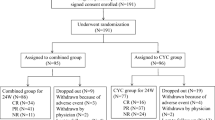
Sixty-two active LN patients (26 induction-resistant and 36 flared) treated with CsA for at least 12 months were included. (Fig. 1). CsA and prednisolone had been prescribed in 40 (64.52%) patients while CsA plus MMF and prednisolone were employed in the other 22 (35.48%) patients. The baseline characteristics of all enrolled patients classified by immunosuppressive are shown in Table 1. At the time of CsA initiation, the dose of prednisolone was increased to more than 20 mg per day in 28 patients (IncPred) and was not increased in the other 34 (StaPred).
Mean age of all enrolled patients was 35.11 ± 10.93 years, and most (91.9%) were female. Minimum dose of CsA used was 50 mg/day, maximum dose was 200 mg/day, and mean CsA daily dose at last visit was 102.8 ± 50.43 mg (1.73 ± 0.91 mg/kg/day). Patients who were treated with CsA and prednisolone dual therapy received higher doses of CsA (127.27 ± 54.15 mg/day) than those who received the triple therapy of CsA plus MMF and prednisolone (97.55 ± 48.54 mg/day) (p = 0.05). (Table 2).
Before CsA initiation, 4 out of 26 patients with induction-resistant LN did not achieve at least partial renal remission after intravenous cyclophosphamide (IVCY) induction treatment and 3 out of 26 patients could not achieve at least partial renal remission after MMF induction treatment. Before CsA initiation, 12 out of 26 patients with induction-resistant LN had partial remission after the IVCY induction therapy, and 7 out of 26 patients had partial remission after MMF induction treatment; however, proteinuria was still higher than 1 g per day. Before CsA initiation, 32 out of 36 patients with flared LN had clinical flare during azathioprine maintenance while the remaining 4 had clinical flare during MMF maintenance. Kidney biopsy was done in 27/62 patients (43.55%). Results of histopathology were LN class III (n = 2, 7.41%), LN class IV (n = 9, 33.33%), pure LN class V (n = 11, 40.74%), LN class III + V (n = 3, 11.11%), and LN class IV + V (n = 2, 7.41%). The result of histopathology subgroup according to immunosuppressive medications (CsA+P or CsA+MMF+P) was shown in Table 1.
Renal response after 6 and 12 months of CsA treatment
Of the 62 studied patients, 27 (43.55%) had nephrotic range proteinuria at the start of CsA treatment. Reduction of UPCR to lower than 50% from baseline was observed 3 months after CSA was initiated. After 12 months of CsA treatment, UPCR in patients with induction-resistant LN and in patients with flared LN had decreased significantly from the baseline regardless of method of CsA being used as CsA and prednisolone or CsA plus MMF and prednisolone (Table 3). Fifty-six LN patients (90.32%) had CR at 12 months after CsA treatment while three (4.84%) had PR (two in CsA and prednisolone group and one in CsA plus MMF and prednisolone group). Mean MMF dose of induction-resistant LN patients was significantly higher than MMF dose used in flared LN patients (2250 ± 323.22 mg/day and 1500 ± 534.52 respectively; p value 0.005). Three patients (4.84%) had treatment failure; all of them had been treated with CsA plus MMF and prednisolone and kidney biopsy showed LN class III+V and LN class III+V and pure LN class V, respectively.
A total of 11 patients had biopsy confirming pure LN class V. Of these 11 patients, CsA was initiated due to induction-resistant LN in 7 patients and LN flared in the other 4. Six of these 11 patients were treated with CsA and prednisolone, and 5 of these 11 patients were treated with CsA plus MMF and prednisolone. The treatment outcomes for patients with pure LN class V were very good, with 9/11 achieving CR. Of the two LN class V patients who failed to have CR, one had PR at 12 months and the remaining one had treatment failure (no CR or PR). In addition, mean dose of prednisolone can be significantly reduced from the baseline of 28.18 ± 16.17 mg/day to 8.64 ± 2.34 mg/day at twelfth month (p < 0.001).
SLE disease activity, dose of prednisolone, and non-renal manifestation
Mean disease activity (mSLEDAI2K) decreased significantly from 23.97 ± 4.29 at baseline to 1.23 ± 4.71 after 12 months of CSA treatment (p < 0.001). In IncPred group, mSLEDAI2K decreased from 24.04 ± 3.66 at baseline to 0.68 ± 3.59 at 12 months (p < 0.001), while in StaPred group, it declined from 23.91 ± 4.81 to 1.68 ± 5.47 at 12 months (p < 0.001). Mean dose of prednisolone was significantly reduced from 33.21 ± 15.23 mg/day at the time of CsA initiation to 9.02 ± 2.91 at 12 months in IncPred group (p < 0.001). Mean dose of prednisolone was also significantly reduced from 13.24 ± 5.13 at baseline to 7.74 ± 3.10 mg/day, at twelfth month in StaPred group (p < 0.001). In addition, there was no statistical significance in prednisolone dose difference at 12 months between IncPred and StaPred group (9.02 ± 2.91 versus 7.74 ± 3.10 mg/day) (p = 0.58).
Anemia was also alleviated in both subgroups; this was noted from the 4th week after treatment with CsA. Hb increased significantly from 11.85 ± 1.60 mg/dl at baseline to 12.76 ± 2.71 mg/dl (p < 0.001) at 12 months in CsA and prednisolone group. A similar significant improvement was also observed in CsA plus MMF and prednisolone group (Hb increased from 12.13 ± 1.68 mg/dl at baseline to 13.11 ± 0.89 mg/dl at 12 months) (p = 0.034). Improvements in other non-renal activities including discoid LE (DLE) (n = 13), alopecia (n = 2), sub-acute cutaneous LE (SCLE) (n = 1), panniculitis (n = 1), cutaneous vasculitis (n = 1), arthritis (n = 6), active AIHA (n = 4), and thrombocytopenia (n = 1) were also observed during the course of CsA treatment.
Adverse reactions
Adverse events were observed in 17 patients. These events were increased serum creatinine of more than 30% (n = 2), new-onset hypertension (n = 3), numbness (n = 1), gingival hyperplasia (n = 2), and new-onset hyperlipidemia (n = 4). Serum Cr returned to baseline spontaneously in one patient and after CsA dose reduction in another. All hypertensive episodes were well controlled with antihypertensive agents. Severe infections, defined as requiring hospitalization or discontinuation of the immunosuppressant, did not occur during CsA treatment in our cohort. Only seven minor infectious events were reported including five upper respiratory tract infections, one acute infectious diarrhea, and one urinary tract infection. Adverse events classified by immunosuppressive drugs used were shown in Table 4. No patients had to discontinue CsA due to adverse events.
Discussion
Calcineurin inhibitors are among the rescue therapies recommended by the ACR for LN patients whose induction therapy fails or who have relapse during maintenance treatment [4]. Currently, CSA and tacrolimus are the two most common calcineurin inhibitors used in clinical practice. CsA was introduced for the treatment of SLE in the late 1990s [16,17,18] and has several advantages over conventional treatment for LN. These include a possible lower carcinogenic effect [19] and ability to suppress hepatitis C viral replication both in vitro and in vivo, and the fact that it has little or no teratogenic effects [20, 21]. However, one of the greatest concerns about the use of CsA in SLE is its adverse reactions; in particular, a potential dose-related decrease in GFR and uncontrolled arterial hypertension, and these have limited its use [22]. In patients with autoimmune diseases, a poor correlation between CsA blood levels and its clinical effects has been noted; thus, the monitoring of CsA blood level is not routinely required [23, 24].
Information about the efficacy of CsA for the treatment of induction-resistant or flared LN is limited. Ogawa et al. [25] administered CsA (target level 80–150 ng/ml) to 59 patients who found unsatisfactory clinical improvement or recurrent exacerbation of disease activity after 12 weeks of initial therapy which included glucocorticoids alone, cyclophosphamide (daily or pulse therapy), azathioprine, methotrexate, or mizoribine. They found clinical remission in 16 of 26 patients (61%), and the mean SLEDAI score of treated patients fell from 8.6 ± 5.3 to 4.4 ± 2.5 after CsA treatment; however, almost one third (32.2%) of treated patients had hypertension or renal insufficiency resulting in CsA discontinuation in up to 14% of treated patients. In contrast, our study has shown that CsA can be effectively used to treat active LN in several clinical scenarios including induction-resistant and flared LN. In addition, we have shown that our approach of utilizing low-dose CsA in conjunction with dose titration is a practical method for treatment of active LN. This method is associated with both good efficacy and tolerability, although close monitoring of several adverse events that may occur during the treatment is essential. The mean dose of CsA in our study (1.73 ± 0.91 mg/kg/day) is, to our knowledge, the lowest of any study performed to date, but it was associated with a very good CR rate (90.32%) at 12 months. In addition, we have shown in our cohort that this approach does not trigger any incidence of permanent decreased GFR or uncontrolled arterial hypertension.
A recent study showed that glucocorticoid-related damage can be decreased and cardiovascular complications limited by a strategy of reducing the dose of prednisolone for the treatment of SLE [26]. In order to monitor the need to increase glucocorticoids dose in active LN in patients who were treated with CsA, we classified them into two subgroups. We found that the dose of prednisolone at the time of CsA initiation was higher in IncPred group (33.21 ± 15.23 mg/day) than in StaPred group (13.24 ± 5.13 mg/day) (p < 0.001); however, at 12 months, the dose of prednisolone was significantly lower in both subgroups (9.02 ± 2.91 mg/day in IncPred group and 7.74 ± 3.10 mg/day in StaPred group) and was not significantly different between the two subgroups (p = 0.58). This information supports the hypothesis that CsA treatment is associated with steroid-sparing effects [11]. However, further studies are required to determine the optimal dose of prednisolone to be used with CsA for the treatment of active LN.
The use of CsA for treating pure LN class V is another interesting issue. The EULAR and the ERA-EDTA recommend calcineurin inhibitors (cyclosporine or tacrolimus) as alternative options to MMF for the initial treatment of membranous lupus nephritis with nephrotic syndrome as well as for non-responders [3, 27, 28]. Austin et al. conducted a randomized control trial of 42 patients with membranous LN, comparing alternate-day prednisolone, intravenous pulse CYC, and cyclosporine. Twelve patients were randomized to receive CsA. The cumulative probability of complete or partial response at 12 months was highest in the cyclosporine group (83%) followed by the CYC (60%) and prednisolone alone (27%) groups. However, the rate of infection (pneumonia and herpes zoster) was found to be a major adverse event of CsA treatment [29]. We did not find any severe infection in our cohort of LN class V who were treated with CsA, and this may be a result of the comparatively low dose of CsA treatment in our study. Despite this low-dose approach, the rate of CR after treatment with CsA in combination with prednisolone with or without MMF appears to be high (9/11).
A multi-target therapy of calcineurin inhibitors, prednisolone, and MMF was recently introduced as a regimen for LN. Most studies of multi-target therapy for LN have focused on the use of tacrolimus in combination with MMF and prednisolone; however, its attendant high rates of infection remain a major cause for concern [22]. Few clinical studies have examined the efficacy of CsA combined with MMF and prednisolone for the treatment of LN. Compared with tacrolimus, CsA has a more favorable effect on glucose metabolism [30] and is associated with lower rates of infection such as BK virus nephropathy. D Jesus et al. examined the effect of CsA plus MMF in six LN patients with refractory disease. Proteinuria was found to be markedly decreased from 2407 mg/24 h to 544 mg/24 h after 6 months. Four patients achieved complete renal response, one had partial response and one failed to respond. Mean prednisolone dose was also able to be reduced from 17.5 to 6 mg/day, and no adverse reaction resulting from this strategy was found [31]. In addition, a recent study evaluated 22 LN patients with persistent proteinuria using glucocorticoid combined with MMF and CsA (approximately 3 mg/kg/day). It was found that 70% of patients responded to treatment; however, infection episodes, including two patients who required hospitalization, are a major concern [32]. Our study shows on a larger scale that low-dose CsA and glucocorticoids in combination with MMF can be used effectively to treat induction-resistant and flared LN. Low-dose CsA using a dose titration technique in our study resulted in good adherence by the patients and very low incidence of infectious complications during the 12-month follow up.
The role of CsA for non-renal active SLE disease is still debated. Our study showed that CsA was beneficial in the treatment of various types of cutaneous lesions (DLE, alopecia, SCLE, panniculitis, and cutaneous vasculitis) and was also effective for treatment of arthritic or hematologic activity (AIHA, thrombocytopenia). Since the incidence of non-renal SLE disease was rather low in our study, we believe that further research is required to confirm the efficacy of CsA for the treatment of non-renal active disease in SLE patients. The use of low-dose CsA is associated with some reversible nephrotoxicity with a follow-up period of 12 months; however, we believe that chronic nephrotoxicity which is associated with a median onset of 3 years is still an important issue [33]. Hence, we propose that after CR has been achieved, the appropriate maintenance therapy after 1 year for patients who respond to CsA should be further evaluated.
Conclusion
Our study showed that CsA is a good alternative treatment for both patients with induction-resistant LN and those with flared LN. The use of lowest effective dose using dose titration while monitoring for adverse events is crucial in maintaining a counterbalance between CsA efficacy and adverse reactions. Low-dose CsA can also be combined with MMF and prednisolone as a type of multi-target therapy to treat active LN.
References
Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC (2012) Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum 41(6):830–839
Mok CC, Kwok RC, Yip PS (2013) Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum 65(8):2154–2160
Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dorner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JP, Isenberg DA, Kallenberg CG, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT (2012) Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71(11):1771–1782
Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM (2012) American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64(6):797–808
Grootscholten C, Berden JH (2006) Discontinuation of immunosuppression in proliferative lupus nephritis: is it possible? Nephrol Dial Transplant 21(6):1465–1469
Tektonidou MG, Dasgupta A, Ward MM (2016) Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol 68(6):1432–1441
Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ (2008) Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol 3(1):46–53
Mok CC (2016) Pro: the use of calcineurin inhibitors in the treatment of lupus nephritis. Nephrol Dial Transplant 31(10):1561–1566
Xiong W, Lahita RG (2014) Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol 10(2):97–107
Mok CC, Yap DY, Navarra SV, Liu ZH, Zhao MH, Lu L, Takeuchi T, Avihingsanon Y, Yu XQ, Lapid EA, Lugue-Lizardo LR, Sumethkul V, Shen N, Chen SL, Chan TM (2014) Overview of lupus nephritis management guidelines and perspective from Asia. Nephrology (Carlton) 19(1):11–20
Suzuki K, Saito K, Tsujimura S, Nakayamada S, Yamaoka K, Sawamukai N, Iwata S, Nawata M, Nakano K, Tanaka Y (2010) Tacrolimus, a calcineurin inhibitor, overcomes treatment unresponsiveness mediated by P-glycoprotein on lymphocytes in refractory rheumatoid arthritis. J Rheumatol 37(3):512–520
Chighizola CB, Ong VH, Meroni PL (2017) The use of cyclosporine a in rheumatology: a 2016 comprehensive review. Clin Rev Allergy Immunol 52(3):401–423
Moroni G, Doria A, Ponticelli C (2009) Cyclosporine (CsA) in lupus nephritis: assessing the evidence. Nephrol Dial Transplant 24(1):15–20
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725
Uribe AG, Vila LM, McGwin G Jr, Sanchez ML, Reveille JD, Alarcon GS (2004) The Systemic Lupus Activity Measure-revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol 31(10):1934–1940
Tokuda M, Kurata N, Mizoguchi A, Inoh M, Seto K, Kinashi M, Takahara J (1994) Effect of low-dose cyclosporin a on systemic lupus erythematosus disease activity. Arthritis Rheum 37(4):551–558
Caccavo D, Lagana B, Mitterhofer AP, Ferri GM, Afeltra A, Amoroso A, Bonomo L (1997) Long-term treatment of systemic lupus erythematosus with cyclosporin A. Arthritis Rheum 40(1):27–35
Dostal C, Tesar V, Rychlik I, Zabka J, Vencovsky J, Bartunkova J, Stejskalova A, Tegzova D (1998) Effect of 1 year cyclosporine A treatment on the activity and renal involvement of systemic lupus erythematosus: a pilot study. Lupus 7(1):29–36
Borigini MJ, Paulus HE (1995) Innovative treatment approaches for rheumatoid arthritis. Combination therapy. Baillieres Clin Rheumatol 9(4):689–710
Ishii N, Watashi K, Hishiki T, Goto K, Inoue D, Hijikata M, Wakita T, Kato N, Shimotohno K (2006) Diverse effects of cyclosporine on hepatitis C virus strain replication. J Virol 80(9):4510–4520
Biggioggero M, Borghi MO, Gerosa M, Trespidi L, Cimaz R, Meroni PI (2007) Immune function in children born to mothers with autoimmune diseases and exposed in utero to immunosuppressants. Lupus 16(8):651–656
Fernandez Nieto M, Jayne DR (2016) Con: the use of calcineurin inhibitors in the treatment of lupus nephritis. Nephrol Dial Transplant 31(10):1567–1571
Griffiths B, Emery P (2001) The treatment of lupus with cyclosporin A. Lupus 10(3):165–170
Ryan C, Amor KT, Menter A (2010) The use of cyclosporine in dermatology: part II. J Am Acad Dermatol 63(6):949–997
Ogawa H, Kameda H, Amano K, Takeuchi T (2010) Efficacy and safety of cyclosporine A in patients with refractory systemic lupus erythematosus in a daily clinical practice. Lupus 19(2):162–169
Ruiz-Arruza I, Lozano J, Cabezas-Rodriguez I, Medina JA, Ugarte A, Erdozain JG, Ruiz-Irastorza G (2018) Restrictive use of oral glucocorticoids in systemic lupus erythematosus and prevention of damage without worsening long-term disease control: an observational study. Arthritis Care Res 70(4):582–591
Radhakrishnan J, Kunis CL, D'Agati V, Appel GB (1994) Cyclosporine treatment of lupus membranous nephropathy. Clin Nephrol 42(3):147–154
Hallegua D, Wallace DJ, Metzger AL, Rinaldi RZ, Klinenberg JR (2000) Cyclosporine for lupus membranous nephritis: experience with ten patients and review of the literature. Lupus 9(4):241–251
Austin HA 3rd, Illei GG, Braun MJ, Balow JE (2009) Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 20(4):901–911
Maes BD, Vanrenterghem YF (2004) Cyclosporine: advantages versus disadvantages vis-a-vis tacrolimus. Transplant Proc 36(2 Suppl):40S–49S
Jesus D, Rodrigues M, da Silva JAP, Ines L (2018) Multitarget therapy of mycophenolate mofetil and cyclosporine A for induction treatment of refractory lupus nephritis. Lupus 27(8):1358–1362
Kasitanon N, Boripatkosol P, Louthrenoo W (2018) Response to combination of mycophenolate mofetil, cyclosporin A and corticosteroid treatment in lupus nephritis patients with persistent proteinuria. Int J Rheum Dis 21(1):200–207
Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RD (2004) Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation 78(4):557–565
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the investigating hospital.
Disclosure
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sumethkul, K., Kitumnuaypong, T., Angthararak, S. et al. Low-dose cyclosporine for active lupus nephritis: a dose titration approach. Clin Rheumatol 38, 2151–2159 (2019). https://doi.org/10.1007/s10067-019-04469-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04469-6