Abstract
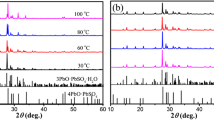
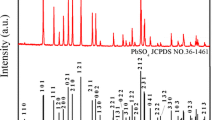
To make full and economic use of the spent lead acid batteries (LABs), we have invented a novel route to separate their negative electrode material from positive one, which are respectively used to fabricate α-PbO for new LABs. This paper reports preparation and electrochemical property of α-PbO from the spent negative material which is compose of PbSO4 (the major phase) and Pb (the minor phase). To make things simpler, pure PbSO4 is firstly used as the model compound and desulfated with (NH4)2CO3 to obtain PbCO3, which is then calcined in air at different temperatures to produce PbO. At 450 °C, the calcination produces pure α-PbO that discharges a capacity of 98.6 mAh g−1 at the current density of 120 mA g−1 after 50 charging and discharging cycles of 100 % DOD. By using the same procedures, the real spent negative powder is also treated to produce pure α-PbO, which discharges a similar capacity of 100 mAh g−1 at 120 mA g−1. This is 25 % higher than that of industrial leady oxide. These results show that the small amount of metallic lead has little effect on the treatment.














Similar content being viewed by others
References
Pavlov D (2011) Lead-acid batteries: science and technology. Elsevier, Amsterdam
Yang JK, Zhu XF, Kumar RV (2011) Ethylene glycol-mediated synthesis of PbO nanocrystal from PbSO4: a major component of lead paste in spent lead acid battery. Mater Chem Phys 131:336–342
Shapira R, Nessim GD, Zimrin T et al (2013) Towards promising electrochemical technology for load leveling applications: extending cycle life of lead acid batteries by the use of carbon nano-tubes (CNTs). Energy Environ Sci 6:587–594
Pan JQ, Sun YZ, Li W et al (2013) A green lead hydrometallurgical process based on a hydrogen-lead oxide fuel cell. Nat Commun 4:2178
Zhang WL, Lin HB, Lu HY et al (2015) On the electrochemical origin of the enhanced charge acceptance of the lead-carbon electrode. J Mater Chem A 3:4399–4404
Ma YJ, Qiu KQ (2015) Recovery of lead from lead paste in spent lead acid battery by hydrometallurgical desulfurization and vacuum thermal reduction. Waste Manag 40:151–156
Shu YH, Ma C, Zhu LG et al (2015) Leaching of lead slag component by sodium chloride and diluted nitric acid and synthesis of ultrafine lead oxide powders. J Power Sources 281:219–226
Gao PR, Liu Y, Bu XF et al (2013) Solvothermal synthesis of α-PbO from lead dioxide and its electrochemical performance as a positive electrode material. J Power Sources 242:299–304
Ferracin LC, Chacon-Sanhueza AE, Davoglio RA et al (2002) Lead recovery from a typical Brazilian sludge of exhausted lead-acid batteries using an electrohydrometallurgical process. Hydrometallurgy 65:137–144
Ellis TW, Mirza AH (2010) The refining of secondary lead for use in advanced lead-acid batteries. J Power Sources 195:4525–4529
Blair TL (1998) Lead oxide technology—past, present, and future. J Power Sources 73:47–55
Lei LX (2009) Method for waste lead-acid cell resourcization and lead-acid cell cyclic production. CN-Patent:101488597
Lei LX, Gao PR, Dai Y (2011) Recycle method of waste lead-acid cell. CN-Patent: 102263309
Yang DN, Liu JW, Wang Q et al (2014) A novel ultrafine leady oxide prepared from spent lead pastes for application as cathode of lead acid battery. J Power Sources 257:27–36
Sonmez MS, Kumar RV (2009) Leaching of waste battery paste components. Part 1: lead citrate synthesis from PbO and PbO2. Hydrometallurgy 95:53–60
Sonmez MS, Kumar RV (2009) Leaching of waste battery paste components. Part 2: leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution. Hydrometallurgy 95:82–86
Zhu XF, Li L, Sun XJ et al (2012) Preparation of basic lead oxide from spent lead acid battery paste via chemical conversion. Hydrometallurgy 117:24–31
Gao PR, Lv WX, Zhang R et al (2014) Methanothermal treatment of carbonated mixtures of PbSO4 and PbO2 to synthesize α-PbO for lead acid batteries. J Power Sources 248:363–369
Li L, Zhu XF, Yang DN et al (2012) Preparation and characterization of nano-structured lead oxide from spent lead acid battery paste. J Hazard Mater 203–204:274–282
Yang JK, Kumar RV, Singh DP (2012) Combustion synthesis of PbO from lead carboxylate precursors relevant to developing a new method for recovering components from spent lead-acid batteries. J Chem Technol Biotechnol 87:1480–1488
Li L, Hu YC, Zhu XF et al (2013) Lead citrate precursor route to synthesize nanostructural lead oxide from spent lead acid battery paste. Mater Res Bull 48:1700–1708
Zhu XF, He X, Yang JK et al (2013) Leaching of spent lead acid battery paste components by sodium citrate and acetic acid. J Hazard Mater 250–251:387–396
Zhu XF, Yang JK, Gao LX et al (2013) Preparation of lead carbonate from spent lead paste via chemical conversion. Hydrometallurgy 134:47–53
Karami H, Karimi MA, Haghdar S (2008) Synthesis of uniform nano-structured lead oxide by sonochemical method and its application as cathode and anode of lead-acid batteries. Mater Res Bull 43:3054–3065
Gong Y, Dutrizac JE, Chen TT (1992) The reaction of anglesite (PbSO4) crystals with sodium-carbonate solutions. Hydrometallurgy 31:175–199
Sajadi SAA, Alamolhoda AA (2006) Synthesis and properties of lead oxide carbonate. Inorg Mater 42:1099–1103
Wang YG, Lin XH, Zhang H et al (2013) Selected-control hydrothermal growths of α- and β-PbO crystals and orientated pressure-induced phase transition. CrystengComm 15:3513–3516
Liu Y, Gao PR, Bu XF et al (2014) Nanocrosses of lead sulphate as the negative active material of lead acid batteries. J Power Sources 263:1–6
Pavlov D (1968) Processes in solid-state at anodic-oxdation of a lead electrode in H2SO4 solution and their dependence on oxide structure and properties. Electrochim Acta 23:845–854
Zhang B, Zhong JH, Li WJ et al (2010) Transformation of inert PbSO4 deposit on the negative electrode of a lead-acid battery into its active state. J Power Sources 195:4338–4343
Kowal J, Budde-Meiwes H, Sauer DU (2012) Interpretation of processes at positive and negative electrode by measurement and simulation of impedance spectra. Part I: inductive semicircles. J Power Sources 207:10–18
Guo YL (1992) A new potential-pH diagram for an anodic film on Pb in H2SO4. J Electrochem Soc 139:2114–2120
Chen T, Huang H, Ma HY et al (2013) Effects of surface morphology of nanostructured PbO2 thin films on their electrochemical properties. Electrochim Acta 88:79–85
Hong B, Jiang LX, Xue HT et al (2014) Characterization of nano-lead-doped active carbon and its application in lead-acid battery. J Power Sources 270:332–341
Saravanan M, Ganesan M, Ambalavanan S (2014) An in situ generated carbon as integrated conductive additive for hierarchical negative plate of lead-acid battery. J Power Sources 251:20–29
Zou XP, Kang ZX, Shu D et al (2015) Effects of carbon additives on the performance of negative electrode of lead-carbon battery. Electrochim Acta 151:89–98
Niya SMR, Hejabi M, Gobal F (2010) Estimation of the kinetic parameters of processes at the negative plate of lead-acid batteries by impedance studies. J Power Sources 195:5789–5793
Hong B, Jiang LX, Hao KT et al (2014) Al/Pb lightweight grids prepared by molten salt electroless plating for application in lead-acid batteries. J Power Sources 256:294–300
Pavlov D, Rogachev T, Nikolov P et al (2009) Mechanism of action of electrochemically active carbons on the processes that take place at the negative plates of lead-acid batteries. J Power Sources 191:58–75
Acknowledgments
The authors would like to thank the Department of Science and Technology, Jiangsu Province (BY2013073-03), Huafu High Technology Energy Storage Co., Ltd., Jiangsu Key Laboratory for Advanced Metallic Materials (BM2007204), and Analytical Test Fund of Southeast University (201226) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Ma, B., Fu, Y. et al. Electrochemical property of α-PbO prepared from the spent negative powders of lead acid batteries. J Solid State Electrochem 21, 35–46 (2017). https://doi.org/10.1007/s10008-016-3333-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3333-1