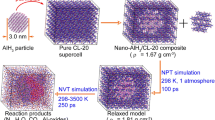
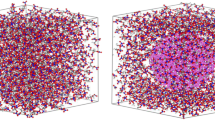
Abstract
The thermal decomposition of pure nitromethane (NM) and NM/nano-aluminum (Al) composites was simulated by reactive molecular dynamics with ReaxFF-lg corrected force field parameters. The initial decomposition pathway of NM molecules in pure NM is C–N bond rupture. However, NM is decomposed early by the initial pathway of N–O bond rupture when it mixes with nano-Al because of the strong attraction of Al to O. The decomposition process of NM/nano-Al can be divided into three stages: adsorption, slow decomposition, and rapid decomposition. The addition of nano-Al particles decreases the energy barrier in decomposition, increases the released energy, and reduces the decomposition temperature of NM. Adding 3% Al to the explosive can make the detonation pressure 3.083% higher than that of pure system. Compared with pure NM, the energy barrier of 16% Al composite is 25.63 kcal/mol lower and the energy released is 22.99 kcal/mol more. There is an optimal amount of Al contents being added to the NM composite by which the largest total numbers of gaseous products (N2, H2O, and CO2) are released. The effect of Al additives on CO2 production is the most obvious. The maximum detonation pressure can be achieved by adding an appropriate amount of nano-Al, which is similar to the experimental results.

Graphical abstract














Similar content being viewed by others
References
Bernstein J (2018) Ab initio study of energy transfer rates and impact sensitivities of crystalline explosives. J. Chem. Phys. 148(8):084502
Dilmaç AM, Spuling E, Meijere A, Bräse S (2017) Propellanes—from a chemical curiosity to “explosive” materials and natural products. Angew. Chem. Int. Edit. 56(21):5684–5718
Watkins EB, Velizhanin KA, Dattelbaum DM, Gustavsen RL, Aslam TD, Podlesak DW, Huber RC, Firestone MA, Ringstrand BS, Willey TM, Bagge-Hansen M, Hodgin RL, Lauderbach L, Buuren A, Sinclair N, Rigg PA, Seifert S, Gog T (2017) Evolution of carbon clusters in the detonation products of the triamino-trinitro-benzene (TATB)-based explosive PBX 9502. J. Phys. Chem. C 121(41):23129–23140.4
Mbugua A, Satija A, Lucht RP, Bane S (2020) Ignition and combustion characterization of single nitromethane and isopropyl nitrate monopropellant droplets under high-temperature and quasi-steady conditions. Combust Flame 212:295–308
Appalakondaiah S, Vaitheeswaran G, Lebegue S (2013) A DFT study on structural, vibrational properties, and quasiparticle band structure of solid nitromethane. J. Chem. Phys. 138(18):184705
Chang J, Lian P, Wei DQ, Chen XR, Zhang QM, Gong ZZ (2010) Thermal decomposition of the solid phase of nitromethane: ab initio molecular dynamics simulations. Phys. Rev. Lett. 105(22):229902
Shrestha KP, Vin N, Hebinet O, Seidel L, Battin-Leclerc F, Zeuch T, Mauss F (2020) Insights into nitromethane combustion from detailed kinetic modeling-pyrolysis experiments in jet-stirred and flow reactors. Fuel 261:116349
Han SP, van Duin ACT, Goddard WA, Strachan A (2011) Thermal decomposition of condensed-phase nitromethane from molecular dynamics from ReaxFF reactive dynamics. J. Phys. Chem. B 115(20):6534–6540
Rom N, Zybin SV, van Duin ACT, Goddard WA, Zeiri Y, Katz G, Kosloff R (2011) Density-dependent liquid nitromethane decomposition: molecular dynamics simulations based on ReaxFF. J. Phys. Chem. A 115(36):10181–10202
Manaa MR, Reed EJ, Fried LE, Galli G, Gygi F (2004) Early chemistry in hot and dense nitromethane: molecular dynamics simulations. J. Chem. Phys. 120(21):10146–10153
Zhong M, Qin H, Liu QJ, Jiang CL, Zhao F, Shang HL, Liu FS, Tang B (2019) A systematic study of the surface structures and energetics of CH3NO2 surfaces by first-principles calculations. J. Mol. Model. 25(6):164
Mei Z, Li CF, Zhao FQ, Xu SY, Ju XH (2019) Reactive molecular dynamics simulation of thermal decomposition for nano-AlH3/TNT and nano-AlH3/CL-20 composites. J. Mater. Sci. 54(9):7016–7027
Mei Z, Li CF, Zhao FQ, Xu SY, Ju XH (2018) Reactive molecular dynamics simulation of thermal decomposition for nano-aluminized explosives. Phys. Chem. Chem. Phys. 20(46):29341–29350
Li CF, Mei Z, Zhao FQ, Xu SY, Ju XH (2018) Molecular dynamic simulation for thermal decomposition of RDX with nano-AlH3 particles. Phys. Chem. Chem. Phys. 20(20):14192–14199
Zhou ZQ, Chen JG, Yuan HY, Nie JX (2017) Effects of aluminum particle size on the detonation pressure of TNT/Al. Propell Explos Pyrot 42(12):1401–1409
He N, Xiang C, Li W, Zhang Q (2018) Experimental study on deflagrating characteristics of nitromethane-aluminum powder. Acta Armamentarii. 39(1):111–117
Wu HJ, Wang XJ, Zhao XL, Wu GD (2009) The study of fast reaction of nitromethane calytized by nano-aluminum. J Atom Mol Phys 26(5):896–902
Shun YY, Zhao YH, Guo HJ, Tian XL, Hou H (2020) Early stages of precipitation in gamma’ phase of a Ni-Al-Ti model alloy: phase-field and first-principles study. Sci. Adv. Mater. 12(5):746–754
Narayan A (2020) Effect of strain and doping on the polar metal phase in LiOsO3. J Phys-Condens mat 32(12):125501
Peng Q, Rahul WGY, Liu GR, Grimme S, De S (2015) Predicting elastic properties of β-HMX from first-principles calculations. J. Phys. Chem. B 119(18):5896–5903
van Duin ACT, Dasgupta S, Loarant F, Goddard WA (2011) ReaxFF: a reactive force field for hydrocarbons. J. Phys. Chem. A 105(41):9396–9409
Chenoweth K, van Duin ACT, Persson P, Cheng MJ, Oxgaard J, Goddard WA (2008) Development and application of a ReaxFF reactive force field for oxidative dehydrogenation on vanadium oxide catalysts. J. Phys. Chem. A 112(37):14645–14654
Guan YL, Lou JP, Liu R, Ma HX, Song JR (2020) Reactive molecular dynamics simulation on thermal decomposition of n-heptane and methylcyclohexane initiated by nitroethane. Fuel 261:116447
Miao F, Cheng XL (2019) Effect of electric field on polarization and decomposition of RDX molecular crystals: a ReaxFF molecular dynamics study. J. Mol. Model. 26(1):2
Vo T, Reeder B, Damone A, Newell P (2019) Effect of domain size, boundary, and loading conditions on mechanical properties of amorphous silica: a reactive molecular dynamics study. Nanomaterials. 10(1):54
Stukowski A (2010) Visualization and analysis of atomistic simulation data with OVITO—the Open Visualization Tool. Model Sim Mater Sci Eng 18(1):015012
Nakamura T, Kawamoto S, Shinoda W (2015) Precise calculation of the local pressure tensor in Cartesian and spherical coordinates in LAMMPS. Comput. Phys. Commun. 190:120–128
Ye CC, Ju XH, Zhao FQ, Xu SY (2012) Adsorption and decomposition mechanism of 1,1-Diamino-2,2-dinitroethylene on Al(111) surface by periodic DFT calculations. Chinese J Chem 30(10):2539–2548
Ye CC, Zhao FQ, Xu SY, Ju XH (2013) Adsorption and decomposition mechanism of hexogen (RDX) on Al(111) surface by periodic DFT calculations. J. Mol. Model. 19(6):2451–2458
Ye CC, Zhao FQ, Xu SY, Ju XH (2013) Density functional theory studies on adsorption and decomposition mechanism of FOX-7 on Al-13 clusters. Can. J. Chem. 91(12):1207–1212
Ye CC, An Q, Xu SY, Ju XH (2017) Adsorption and decomposition of HMX and CL-20 on Al(111) surface by DFT investigation. Surf. Interface Anal. 49(5):441–449
Ao W, Liu X, Rezaiguia H, Liu H, Wang ZX, Liu PJ (2017) Aluminum agglomeration involving the second mergence of agglomerates on the solid propellants burning surface: experiments and modeling. Acta Astronaut 136:219–229
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Wang, XK., Zhao, Y., Zhao, FQ. et al. Reactive molecular dynamics simulation of thermal decomposition for nitromethane/nano-aluminum composites. J Mol Model 26, 300 (2020). https://doi.org/10.1007/s00894-020-04562-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04562-7