Abstract
Using molecular dynamics method, the ion rejection and water flow inside flexible disjoint carbon-based channels were examined in the presence of electric fields. The effects of the carbon nanotube diameters and field magnitude on the nano-channel efficiency were investigated. It was observed that water flow through the filter was modified by increasing the radius of nanotubes, while the salt rejection was reduced. The particles’ behaviors inside the channel were described in view of Van der Waals interactions between the water molecules, ions, and carbon atoms. Furthermore, the results indicated that the ion rejection and water flow were increased under the application of proper magnitude of electric fields.

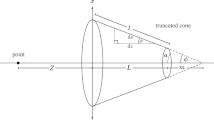
Using MD simulation method, a disjoint CNT-based filter was designed to produce freshwater from a NaCl solution by the aid of external electric field. It was observed that the filter operation was significantly affected by channel structural parameters and amount of applied electric fields.



Similar content being viewed by others
References
Kannam SK, Todd BD, Hansen JS, Daivis PJ (2013) How fast does water flow in carbon nanotubes? J Chem Phys 138:094701
Wu K, Chen Z, Li J, Li X, Xu J, Dong X (2017) Wettability effect on nanoconfined water flow. PNAS 114:3358
Kargar M, Lohrasebi A (2017) Deformation of water nano-droplets on graphene under the influence of constant and alternative electric fields. PhysChemChemPhys 19:26833
Lohrasebi A, Koslowski T (2019) Modeling water purification by an aquaporin-inspired graphene-based nano-channel. J Mol Model 25:280
Ang EYM, Ng TY, Yeo J, Lin R, Liu Z, Geethalakshmi KR, Toh W (2020) A review on low dimensional carbon desalination and gas separation membrane designs. J Membr Sci 598:117785
Neek-Amal M, Peeters FM, Grigorieva IV, Geim AK (2016) Commensurability effects in viscosity of nanoconfined water. ACS Nano 10(3):3685–3692
Cohen-Tanugi D, Lin LC, Grossman JC (2016) Multilayer nanoporous graphene membranes for water desalination. Nano Lett 16:1027–1033
Kargar M, Khasheii F, Lohrasebi A (2018) Influence of electric fields on the efficiency of multilayer graphene membrane. J Mol Model 24:241
Qiu H, Zeng XC, Guo W (2015) Water in inhomogeneous nanoconfinement: coexistence of multilayered liquid and transition to ice nanoribbons. ACS Nano 9:9877–9884
Joly L, Tocci G, Merabia S, Michaelides A (2016) Strong coupling between nanofluidic transport and interfacial chemistry: how defects reactivity controls liquid–solid friction through hydrogen bonding. J Phys Chem Lett 7(7):1381–1386
Chaban V (2010) Filling carbon nanotubes with liquid acetonitrile. Chem Phys Lett 496(1–3):50–55
Chaban V (2010) Should carbon nanotubes be degasified before filling? Chem Phys Lett 500(1–3):35–40
Chaban V, Prezhdo O (2014) Nanoscale carbon greatly enhances mobility of a highly viscous ionic liquid. ACS Nano 8(8):8190–8197
Wei N, Peng X, Xu Z (2014) Understanding water permeation in graphene oxide membranes. ACS Appl Mater Interfaces 6:5877–5883
Dai H, Xu Z, Yang X (2016) Water permeation and ion rejection in layer-by-layer stacked graphene oxide nanochannels: a molecular dynamics simulation. J Phys Chem C 120:22585–22596
Meng XW, Shen L (2020) Transport between one dimensional disjoint nanochannels. Chem Phys Lett 739:137029
Sahimi M, Ebrahimi F (2019) Efficient transport between disjoint nanochannels by a water bridge. Phys Rev Lett 122:214506
Walther JH, Ritos K, Cruz-Chu ER, Megaridis CM, Koumoutsakos P (2013) Barriers to superfast water transport in carbon nanotube membranes. Nano Lett 13(5):1910–1914
Cheng C, Jiang G, Garvey CJ, Wang Y, Simon GP, Liu JZ, Li D (2016) Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci Adv 2:e1501272
Hong S, Constans C, Surmani Martins MV, Seow YC, Guevara Carrió JA, Garaj S (2017) Scalable graphene-based membranes for ionic sieving with ultrahigh charge selectivity. Nano Lett 17:728–732
Ilie A, Crampin S, Karlsson L, Wilson M (2012) Repair and stabilization in confined nanoscale systems - inorganic nanowires within single-walled carbon nanotubes. Nano Res 5:833
Choe H, Hong MH, Seo Y, Lee K, Kim G, Cho Y, Ihm J, Jhe W (2005) Formation, manipulation, and elasticity measurement of a nanometric column of water molecules. Phys Rev Lett 95:187801
An S, Jhe W (2019) Nanopipette/nanorod-combined quartz tuning fork–atomic force microscope. Sensors 19(8):1794
Jin C, Suenaga K, Iijima S (2008) Plumbing carbon nanotubes. Nat Nanotechnol 3:17
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117:1–19
Tuart SJ, Tutein AB, Harrison JA (2000) A reactive potential for hydrocarbons with intermolecular interactions. J Chem Phys 112:6472–6486
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Akbarian D, Yilmaz DE, Cao Y, Ganesh P, Dabo I, Munro J, Ginhoven RV, van Duin ACT (2019) Understanding the influence of defects and surface chemistry on ferroelectric switching: a ReaxFF investigation of BaTiO3. Phys Chem Chem Phys 21(33):18240–18249
Akbarian D, Hamedi H, Damirchi B, Yilmaz DE, Penrod K, Hunter Woodward WH, Moore J, Lanagan MT, van Duin ACT (2019) Atomistic-scale insights into the crosslinking of polyethylene induced by peroxides. Polymer 183:121901
van Duin ACT, Dasgupta S, Lorant F, Goddard WA (2001) ReaxFF: a reactive force field for hydrocarbons. J Phys Chem A 105:9396–9409
Allen M, Tildesley DJ (1987) Computer simulation of liquids. Oxford University Press, New York
Conway BE (1981) Ionic hydration in chemistry and biophysics. Elsevier Science Ltd
Kargar M, Lohrasebi A (2019) Water flow modeling through the graphene-based nanochannel: theory and simulation. Phys Chem Chem Phys 21:3304–3309
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rizi, S.H., Lohrasebi, A. Water distillation modeling by disjoint CNT-based channels under the influence of external electric fields. J Mol Model 26, 236 (2020). https://doi.org/10.1007/s00894-020-04492-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04492-4