Abstract
Understanding the mechanism of water and particle transport through thin-film membranes is essential to improve the water permeability and the salt rejection rate of the purification progress. In this research, mimicking from the structure and operation of the aquaporin channel, graphene-based nano-channels were designed to be used as a water filter. The effects of variation of the channel’s main elements, such as the width of the bottleneck and charges attached to the channel on its efficiency, were investigated via molecular dynamics simulations. We observe that the water flow through the channel decreases by increasing the charge, while the ion rejection rate of the channel is enhanced. Moreover, we find that the geometry and shape of the bottleneck part of the channel can affect the channel water flow and its selectivity. Finally, the pressure and the flow velocity in the channel were considered by using finite element models, and the results indicate that they are high at the entrance of the channel. The outcomes of this study can be used to improve the molecular knowledge of water desalination, which might be helpful in designing more efficient membranes.

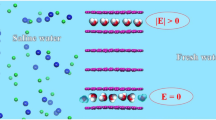
As the piston pushed the solution to pass through the nano-channel, positive and negative ions are remained in the first box, by sensing electric field generated from the attached charges to the bottleneck part of the channel. Atomistic structure of channel is shown in the right part of the figure and the generated electric field is shown in the left part of the figure.






Similar content being viewed by others
References
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red-cell CHIP28 protein. Science 256:385–387
Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y (2000) Structural determinants of water permeation through aquaporin-1. Nature 407:599–605
Tajkhorshid E, Nollert P, Jensen MØ, Miercke LJW, O’Connell J, Stroud RM, Schulten K (2002) Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296:525–530
Vidossich P, Cascella M, Carloni P (2004) Dynamics and energetic of water permeation through the aquaporin channel. Proteins 55:924–931
Aponte-Santamaría C, Fischer G, Båth P, Neutze R, de Groot BL (2017) Temperature dependence of protein-water interactions in a gated yeast aquaporin. Sci. Rep. 7(1):4016
Saboe PO, Rapisarda C, Kaptan S, Hsiao YS, Summers SR, Zorzi RD, Dukovski D, Yu J, de Groot BL, Kumar M, Walz T (2017) Role of pore-lining residues in defining the rate of water conduction by aquaporin-0. Biophys. J. 112(14):953–965
de Groot BL, Grubmuller H (2001) Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science 294:2353–2357
Lohrasebi A, Rafii-Tabar H (2008) Computational modeling of an ion-driven nanomotor. Journal of Molecular Graphics and Modeling 27:116–123
Gong X, Li J, Lu H, Wan R, Li J, Hu J, Fang H (2007) A charge-driven molecular water pump. Nature 2:709–712
Chen M, Zang J, Xiao D, Zhang C, Liu F (2009) Nanopumping molecules via a carbon nanotube. Nano Res. 2:938–944
Lohrasebi A, Feshanjerdi M (2012) A rotary nano ion pump: a molecular dynamics study. J. Mol. Model. 18:4191–4197
Rikhtehgaran S, Lohrasebi A (2015) Water desalination by a designed nano filter of graphene-charged carbon nanotube: a molecular dynamics study. Desalination 365:176–181
Surwade SP, Smirnov SN, Vlassiouk IV, Unocic RR, Veith GM, Dai S, Mahurin SM (2015) Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 10:459–464
Wu K, Chen Z, Li J, Li X, Xu J, Dong X (2017) Wettability effect on nanoconfined water flow. PNAS 114:3358–3363
Cohen-Tanugi D, Grossman JC (2015) Nanoporous graphene as a reverse osmosis membrane: recent insights from theory and simulation. Desalination 366:59–70
Sun P, Wang K, Zhu H (2016) Recent developments in graphene-based membranes: structure. Mass-Transport Mechanism and Potential Applications, Advanced Materials 28:2287–2310
Lohrasebi A, Rikhtehgaran S (2018) Ion separation and water purification by applying external electric field on porous graphene membrane. Nano Res. 11(4):2229–2236
Cohen-Tanugi D, Lin LC, Grossman JC (2016) Multilayer nanoporous graphene membranes for water desalination. Nano Lett. 16:1027–1033
Neek-Amal M, Lohrasebi A, Mousaei M, Radha B, Peeters FM (2018) Fast water flow through graphene nanocapillaries: a continuum model approach involving the microscopic structure of confined water. Appl. Phys. Lett. 113(8):083101–083106
Kargar M, Khasheii F, Lohrasebi A (2018) Influence of electric fields on the efficiency of multilayer graphene membrane. J. Mol. Model. 24:241
Qiu H, Zeng XC, Guo W (2015) Water in inhomogeneous nanoconfinement: coexistence of multilayered liquid and transition to ice nanoribbons. ACS Nano 9:9877–9884
Joly L, Tocci G, Merabia S, Michaelides A (2016) Strong coupling between nanofluidic transport and interfacial chemistry: how defect reactivity controls liquid–solid friction through hydrogen bonding. J. Phys. Chem. Lett. 7(7):1381–1386
Walther JH, Ritos K, Cruz-Chu ER, Megaridis CM, Koumoutsakos P (2013) Barriers to superfast water transport in carbon nanotube membranes. Nano Lett. 13(5):1910–1914
Kargar M, Lohrasebi A (2019) Water flow modeling through the graphene-based nanochannel: theory and simulation. Phys. Chem. Chem. Phys. 21:3304–3309
Chakraborty S, Kumar H, Dasgupta C, Maiti PK (2017) Confined water: structure, dynamics, and thermodynamics. Acc. Chem. Res 50(9):2139–2146
Giri AK, Teixeira F, Natália M, Cordeiro DS (2019) Salt separation from water using graphene oxide nanochannels: a molecular dynamics simulation study. Desalination 460:1–14
Giri AK, Teixeira F, Natália M, Cordeiro DS (2018) Structure and kinetics of water in highly confined conditions: a molecular dynamics simulation study. J. Mole. Liq. 268:625–636
Cheng C, Jiang G, Garvey CJ, Wang Y, Simon GP, Liu JZ, Li D (2016) Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci. Adv. 2:1501272
Hong S, Constans C, Surmani Martins MV, Seow YC, Guevara Carrió JA, Garaj S (2017) Scalable graphene-based membranes for ionic sieving with ultrahigh charge selectivity. Nano Lett. 17:728–732
Zhou X, Wu F, Kou J, Nie X, Liu Y, Lu H (2013) Vibrating-charge-driven water pump controlled by the deformation of the carbon nanotube. J. Phys. Chem. B 117(39):11681–11686
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl 2. J. Exp. Bot. 55(396):411–422
Abraham J, Vasu KS, Williams CD, Gopinadhan K, Su Y, Cherian CT, Dix J, Prestat E, Haigh SJ, Grigorieva IV, Carbone P, Geim AK, Nair RR (2017) Tuneable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 12(6):546–550
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117:1–19
SJ Tuart, AB Tutein, JA Harrison (2000) A reactive potential for hydrocarbons with intermolecular interactions, J. Chem. Phys. 112, 6472–6486
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79:926–935
Allen M, Tildesley DJ (1987) Computer simulation of liquids. Oxford University Press, New York
COMSOL Multiphysics. Key: citeulike: 3255057
Conway BE (1981) Ionic hydration in chemistry and biophysics. Elsevier Science, Ltd.
Zeidel ML, Ambudkar SV, Smith BL, Agre P (1992) Water permeability of asymmetric planar lipid bilayers. Biochemistry 31:7436–7440
Kumar M, Grzelakowski M, Zilles J, Clark M, Meier W (2007) Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl Acad. Sci. 104:20719–20724
Geng, J. et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature514, 612–615 (2014)
Tunuguntla RH et al (2017) Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 357:792–796
Garaj S, Hubbard W, Reina A, Kong J, Branton D, Golovchenko JA (2010) Graphene as a subnanometre trans-electrode membrane. Nature 467:190–194
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 614 kb)
Rights and permissions
About this article
Cite this article
Lohrasebi, A., Koslowski, T. Modeling water purification by an aquaporin-inspired graphene-based nano-channel. J Mol Model 25, 280 (2019). https://doi.org/10.1007/s00894-019-4160-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4160-y