Abstract
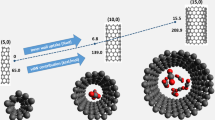
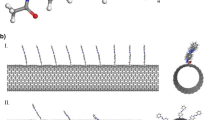
Biological applications of single-walled carbon nanotubes (SWCNTs), including drug delivery, require their functionalization with various functional groups such as peptides. Recently, a biologically compatible peptide (named PW3 with the sequence of NH2-Trp-Val-Trp-Val-Trp-Val-Lys-Lys-COOH) has been introduced as a good candidate for modification of carbon nanotubes due to its high affinity toward the exterior surface of these nano-carriers. In order to optimize the process of SWCNT peptide functionalization, the effects of chirality and diameter of SWCNTs as well as the temperature on PW3 adsorption were systematically investigated using molecular dynamics (MD) simulation. It was found that modification of chiral/zigzag SWCNT by PW3 peptide was more suitable compared with the armchair system due to the strong peptide-nanotube interactions and more water solubility at 310 K which can be well explained by microscopic structural investigations. Regarding the enhanced peptide-chiral nanotube interactions at the low temperature of 277 K, chiral nanotubes can be effective structures for SWCNT functionalization process at reduced temperatures. Our analysis indicated that disrupted PW3 and SWCNT hydration patterns and fewer internal interactions within the peptide could be responsible for the stronger peptide modification of SWCNT at higher temperatures. Additionally, “PW3/SWCNT” systems containing larger tube diameters formed more stable complexes owing to their effective surface area increment.












Similar content being viewed by others
References
De Volder MFL, Tawfick SH, Baughman RH, Hart AJ (2013) Carbon nanotubes: present and future commercial applications. Science 339:535–539
Bandaru PR (2007) Electrical properties and application of carbon nanotube structures. J. Nanosci. Nanotechnol. 7:1239–1267
Maiti UN, Lee WJ, Lee JM, Oh Y, Kim JY, Kim JE, Shim J, Han TH, Kim SO (2014) 25th anniversary article: chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices. Adv. Mater. 26:40–67
Son KH, Hong JH, Lee JW (2016) Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomedicine 11:5163–5185
Mundra RV, Wu X, Sauer J, Dordick JS, Kane RS (2014) Nanotubes in biological applications. Curr. Opin. Biotechnol. 28:25–32
Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH, Pastorin G (2013) Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 65:1964–2015
Antonucci A, Kupis-Rozmyslowicz J, Boghossian AA (2017) Noncovalent protein and peptide functionalization of single-walled carbon nanotubes for biodelivery and optical sensing applications. ACS Appl. Mater. Interfaces 9:11321–11331
Cha I, Hashimotob K, Fujikic T, Yamauchi T, Tsubokawa N (2014) Modification of dispersibility of nanodiamond by grafting of polyoxyethylene and by the introduction of ionic groups onto the surface via radical trapping. Mater. Chem. Phys. 143:1131–1138
Heister E, Neves V, Tilmaciu C, Lipert K, Beltran VS, Coley HM, Silva SRP (2009) Triple functionalization of single-walled carbon nanotubes with doxorubicin, a monoclonal antibody, and a fluorescent marker for targeted cancer therapy. CARBON 47:2152–2160
Liu Z, Fan AC, Rakhra K, Sherlock S, Goodwin A, Chen X, Yang Q, Felsher DW, Dai H (2009) Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angew. Chem. Int. Ed. Engl 48:7668–7672
Zhang Y, Bai Y, Yan B (2010) Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 15:428–435
Liu Z, Sun X, Nakayama-Ratchford N, Dai H (2007) Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 1:50–56
Digge MS, Moon RS, Gattani SG (2012) Application of carbon nanotubes in drug delivery: a review. Int J Pharm Tech Res 4:839–847
Chiu C, Maher MC, Dieckmann GR, Nielsen SO (2010) Molecular dynamics study of a carbon nanotube binding reversible cyclic peptide. ACS Nano 4:2539–2546
Bianco A, Kostarelos K, Prato M (2005) Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 9:674–679
Lacerda L, Bianco A, Prato M, Kostarelos K (2006) Carbon nanotubes as nano medicines: from toxicology to pharmacology. Adv. Drug Deliv. Rev. 58:1460–1470
Chiu C, Dieckmann GR, Nielsen SO (2009) Role of peptide-peptide interactions in stabilizing peptide-wrapped single-walled carbon nanotubes: a molecular dynamics study. Biopolymers 92:156–163
Wu E, Coppens M, Garde S (2015) Role of arginine in mediating protein-carbon nanotube interactions. Langmuir 31:1683–1692
Barzegar A, Mansouri A, Azamat J (2016) Molecular dynamics simulation of non-covalent single-walled carbon nanotube functionalization with surfactant peptides. J. Mol. Graphics Model 64:75–84
Cheng Y, Liu GR, Li ZR, Lu C (2006) Computational analysis of binding free energies between peptides and single-walled carbon nanotubes. Physica A 367:293–304
Xie H, Becraft EJ, Baughman RH, Dalton AB, Dieckmann GR (2008) Ranking the affinity of aromatic residues for carbon nanotubes by using designed surfactant peptides. J. Pept. Sci. 14:139–151
Az’hari S, Ghayeb Y (2014) Effect of chirality, length and diameter of carbon nanotubes on the adsorption of 20 amino acids: a molecular dynamics simulation study. Mol. Simul. 42:392–398
Wang C, Li S, Zhang R, Lin Z (2012) Adsorption and properties of aromatic amino acids on single-walled carbon nanotubes. Nanoscale 4:1146–1153
Rajesh C, Majumder C, Mizuseki H, Kawazoe YA (2009) Theoretical study on the interaction of aromatic amino acids with graphene and single walled carbon nanotube. J. Chem. Phys. 130:124911
Gu Z, Yang Z, Chong Y, Ge C, Weber JK, Bell DR, Zhou R (2015) Surface curvature relation to protein adsorption for carbon-based nanomaterials. Sci. Rep. 5:10886
Raffaini G, Ganazzoli F (2010) Protein adsorption on biomaterial and nanomaterial surfaces: a molecular modeling approach to study non-covalent interactions. J. Appl. Biomater Biomech 8:135–145
Raffaini G, Ganazzoli F (2013) Surface topography effects in protein adsorption on nanostructured carbon allotropes. Langmuir 29:4883–4893
Li Z, Tozer T, Alisaraie L (2016) Molecular dynamics studies for optimization of noncovalent loading of vinblastine on single-walled carbon nanotube. J. Phys. Chem. C 120:4061–4070
http://www.jcrystal.com/products/wincnt/, Nanotube Modeler, JCrystalSoft Ed., 2004–2005
Froimowitz M (1993) HyperChem: a software package for computational chemistry and molecular modeling. Biotechniques 14:1010–1013
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79:926–935
MacKerell AD, Bashford D, Bellott RL, Dunbrack JD, Evanseck MJ, Field S, Fischer J, Gao H, Guo S, Ha D, Joseph-McCarthy L, Kuchnir K, Kuczera FTK, Lau C, Mattos S, Michnick T, Ngo DT, Nguyen B, Prodhom WE, Reiher B, Roux M, Schlenkrich JC, Smith R, Stote J, Straub M, Watanabe J, Wiórkiewicz-Kuczera D, Yin MK (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102:3586–3616
Kalé L, Skeel R, Bhandarkar M, Brunner R, Gursoy A, Krawetz N, Phillips J, Shinozaki A, Varadarajan K, Schulten K (1992) NAMD2: greater scalability for parallel molecular dynamics. J. Comput. Phys. 151:283–312
Feller SE, Zhang Y, Pastor RW, Brooks BR (1995) Constant pressure molecular dynamics simulation: the langvin piston method. J. Chem. Phys. 103:4613–4621
Kolb A, Dunwew B (1999) Optimized constant pressure stochastic dynamics. J. Chem. Phys. 111:4453–4459
Alisaraie L, Tuszynski JA (2011) Determination of noscapine’s localization and interaction with the tubulin-A/B heterodimer. Chem. Biol. Drug Des 78:535–546
Tuszynski J, Craddock TA, Mane J, Barakat K, Tseng C-Y, Gajewski M, Winter P, Alisaraie L, Patterson J, Carpenter E, Wang W, Deyholos MK, Li L, Sun X, Zhang Y, Wong GK (2012) Modeling the yew tree tubulin and a comparison of its interaction with paclitaxel to human tubulin. Pharm. Res. 29:3007–3021
Darden T, York D (1993) Particle mesh Ewald: an N·log (N) method for Ewald sums in large systems. J. Chem. Phys. 98:10089–10092
Lee JH (2006) A study on a boron-nitride nanotube as a gigahertz oscillator. J. Korean Phys. Soc. 49:172–176
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14:33–38
Raffaini G, Ganazzoli F (2004) Molecular dynamics simulation of the adsorption of a fibronectin module on a graphite surface. Langmuir 20:3371–3378
Raffaini G, Ganazzoli F (2003) Simulation study of the interaction of some albumin subdomains with a flat graphite surface. Langmuir 19:3403–3412
Walther JH, Jaffe R, Halicioglu T, Koumoutsakos P (2001) Carbon nanotubes in water: structural characteristics and energetics. J. Phys. Chem. B 105:9980–9987
Tamsyn AH, James MH (2007) Modelling the encapsulation of the anticancer drug cisplatin into carbon nanotubes. Nanotechnology 18:275704
Hilder TA, Hill JM (2008) Probability of encapsulation of paclitaxel and doxorubicin into carbon nanotubes. Micro Nano Lett 3:41–49
Karachevtsev V, Plokhotnichenko A, Karachevtsev M, Leontiev V (2010) Decrease of carbon nanotube UV light absorption induced by π–π-stacking interaction with nucleotide bases. Carbon 48:3682–3691
Wang Y, Ai H (2009) Theoretical insights into the interaction mechanism between proteins and SWCNTs: adsorptions of tripeptides GXG on SWCNTs. J. Phys. Chem. B 113:9620–9627
Fujigaya T, Nakashima N (2015) Non-covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 16:024802
Aihara J (1994) Lack of superaromaticity in carbon nanotubes. J. Phys. Chem. 98:9773–9776
Matsuda Y, Tahir-Kheli J, Goddard WA (2010) Definitive band gaps for single-wall carbon nanotubes. J. Phys. Chem. Lett. 1:2946–2950
Ouyang M, Huang J-H, Cheung CL, Lieber CM (2001) Energy gaps in “metallic” single-walled carbon nanotubes. Science 292:702–705
Ding JW, Yan XH, Cao JX (2002) Analytical relation of band gaps to both chirality and diameter of single-wall carbon nanotubes. Phys. Rev. B 66:073401
Toosy NKA, Raissi H, Zaboli M (2016) Theoretical calculations of intramolecular hydrogen bond of the 2-amino-2, 4, 6-cycloheptatrien-1-one in the gas phase and solution: substituent effects and their positions. J. Theor. Comput. Chem. 15:1650063
Small PA (1953) Some factors affecting the solubility of polymers. J. Appl. Chem. 3:71–80
Hashemzade H, Raissi H (2017) The functionalization of carbon nanotubes to enhance the efficacy of the anticancer drug paclitaxel: a molecular dynamics simulation study. J. Mol. Model. 23:222–231
Shanthi V, Ramanathan K, Sethumadhavan R (2009) Role of the cation-π interaction in therapeutic proteins: a comparative study with conventional stabilizing forces. J Comput Sci Syst Biol 2:51–68
Faujan NH, Karjiban RA, Kashaban I, Basri M, Basri H (2015) Computational simulation of palm kernel oil-based esters nano-emulsions aggregation as a potential parenteral drug delivery system. Arab. J. Chem. https://doi.org/10.1016/j.arabjc.2015.03.003
Lee B, Richards FM (1971) The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55:379–400
Ausaf AS, Hassan I, Islam A, Ahmad FA (2014) Review of methods available to estimate solvent-accessible surface areas of soluble proteins in the folded and unfolded states. Curr. Protein Pept. Sci. 15:456–476
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1651 kb)
Rights and permissions
About this article
Cite this article
Tohidifar, L., Hadipour, N.L. Tracing chirality, diameter dependence, and temperature-controlling of single-walled carbon nanotube non-covalent functionalization by biologically compatible peptide: insights from molecular dynamics simulations. J Mol Model 25, 274 (2019). https://doi.org/10.1007/s00894-019-4154-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4154-9